Zebrafish Keratocyte Explants to Study Collective Cell Migration and Reepithelialization in Cutaneous Wound Healing
Summary
Zebrafish keratocytes migrate in cell sheets from explants and provide an in vitro model for the study of the mechanisms of collective cell migration in the context of epithelial wound healing. These protocols detail an effective way to establish primary explant cultures for use in collective cell migration assays.
Abstract
Due to their unique motile properties, fish keratocytes dissociated from explant cultures have long been used to study the mechanisms of single cell migration. However, when explants are established, these cells also move collectively, maintaining many of the features which make individual keratocytes an attractive model to study migration: rapid rates of motility, extensive actin-rich lamellae with a perpendicular actin cable, and relatively constant speed and direction of migration. In early explants, the rapid interconversion of cells migrating individually with those migrating collectively allows the study of the role of cell-cell adhesions in determining the mode of migration, and emphasizes the molecular links between the two modes of migration. Cells in later explants lose their ability to migrate rapidly and collectively as an epithelial to mesenchymal transition occurs and genes associated with wound healing and inflammation are differentially expressed. Thus, keratocyte explants can serve as an in vitro model for the reepithelialization that occurs during cutaneous wound healing and can represent a unique system to study mechanisms of collective cell migration in the context of a defined program of gene expression changes. A variety of mutant and transgenic zebrafish lines are available, which allows explants to be established from fish with different genetic backgrounds. This allows the role of different proteins within these processes to be uniquely addressed. The protocols outlined here describe an easy and effective method for establishing these explant cultures for use in a variety of assays related to collective cell migration.
Introduction
Studies of cell motility have traditionally focused on individually migrating cells. Keratocytes cultured from fish and amphibian explants have proven to be a powerful model system to study the mechanisms of cell motility using both experimental and mathematical modeling approaches1-6. Experimental work on individually migrating cells is aided by the rapid, smooth gliding motion of the cells, while maintaining a uniform shape, speed, and direction. However, in vivo, cells frequently move collectively while maintaining cell-cell junctions, particularly during embryogenesis, wound healing, and metastasis. Thus, collective cell migration is a growing and increasingly prominent area of research within the field of cell migration. The collective migration of these cells has recently been described7 and it has many unique features which make it a powerful system to study collective cell migration in a wound healing model.
When scales are plucked from an anesthetized adult zebrafish, some epithelial tissue remains attached to the underside of the scale. When cell culture medium is added, these epithelial cells, or keratocytes, migrate rapidly as a collective unit from the scale. The explant culture can be viewed as an epithelial wound healing model, in which the cells migrate away from the explant as they would across the provisional matrix of a wound bed to reestablish an intact epithelium. Recent data suggests that this ex vivo culture mimics many features of in vivo wound healing in adult zebrafish. Wound closure rates8 translate to a migration speed similar to that observed in the ex vivo system7. In addition, in an in vivo wound healing model, treatment with warfarin suggests that hemostasis has no effect on rate of healing, while treatment with hydrocortisone to decrease immune response does not delay reepithelialization8. As the zebrafish does not bleed when the scale is removed from the animal, hemostasis is not a major player. Although we have found immune cells in explants, we have not studied the effects they might have on collective cell migration.
The protocol presented here describes how to establish zebrafish keratocyte explant cultures that ensure consistency and reproducibility for use in many biological assays. These explant cultures are a particularly compelling in vitro model for collective cell migration for several reasons. First, zebrafish keratocytes are primary cells used within hours of establishing explant cultures. Therefore, these cells have not undergone the morphological and gene expression changes associated with passage of primary cells9-13. Second, this system represents a model for the study of reepithelialization in response to wounding14 and, as part of the response to wounding, an epithelial to mesenchymal transition (EMT) process is initiated as evidenced by changes in gene expression and cytoskeletal rearrangements14,15. Thus, the background changes in gene expression and motility which occur in untreated cells have been characterized and provide a context to interpret changes with treatment. Furthermore, the fish keratocyte and the human keratinocyte are functionally equivalent; both are the primary epithelial cells of their respective species and play a key role in epithelial wound healing. Third, adult zebrafish are gaining recognition as a model system for a variety of human diseases16-18 including melanoma and other cancers19-21, cutaneous wound healing8,14 and tissue regeneration22-24.
Technical advantages of this model system are equally compelling. A growing number of mutant and transgenic lines are available from non-profit, centralized facilities. Specifically, the Zebrafish Mutation Project (ZMP) at the Wellcome Trust Sanger Institute aims to create a knockout allele in every protein coding gene in the zebrafish genome. Currently, they have mutated 11,892 genes, approximately 45% of the genome, with 24,088 alleles characterized. In addition, ZMP accepts proposals and will generate knock-outs free of charge. Recent methods in gene knockdown in larval and adult zebrafish25-28 provide flexibility to study the function of developmentally important genes for which transgenic approaches are problematic or impractical.
Due to their extremely rapid rates of motility of ~145 µm/hr shortly after establishment of cultures29, assays can be completed rapidly (usually in 24 hr or less). As experiments can be completed rapidly and cultures grow well at RT, several of the technical hurdles associated with mammalian cell migration assays involving video microscopy are avoided. In addition, in the age of tightening research budgets, zebrafish are easily and inexpensively maintained.
The structure and behavior of the explant is complex. As the fish do not bleed when the scale is removed, it is unlikely that much more than the superficial epidermal layers are removed with the scale. Keratocytes appear to be the predominant cell type in the explant when scales are initially removed from the fish as the vast majority of cells appear to stain with an anti-E-cadherin antibody14. Furthermore, there is no evidence of fibroblasts in the initial culture as judged by the absence of vimentin staining by immunofluorescence14. However, with repeated scale removal, there appear to be numerous neutrophils in the explant (see Video 1). We have identified these cells as neutrophils based on morphological observations of their size, rapidly motility, and their migration out of the cell sheet when exposed to LPS (data not shown). Additionally, these cells stain brightly with an antibody to neutrophil cytosolic factor as well as with a variety of secondary IgG antibodies, which indicates that they have abundant FcRs on their surface (data not shown). Multiple cell layers are present within the explant. Confocal microscopy reveals the presence of a single layer near the leading edge to more than two layers of keratocytes near the scale with neutrophils visible above, below, and between the cell keratocyte sheets.
At early time points, cells on the edge of the multi-layer explant polarize, initiating collective migration of keratocytes from the explant at initial rates of ~145 µm/hr. The area covered by the explant rapidly increases, leading to cell spreading, tension within the sheet, and a decreased rate of advance of the leading edge. Rapid interconversions between leader and follower cells are observed at the leading edge during the formation and closure of spontaneously formed holes within the sheet7. As the EMT process initiated with the explant continues, the sheet fragments and the keratocytes lose their specialized, rapid motility. Gene expression and morphological changes consistent with EMT, wound healing, and inflammatory responses occur within 7 days of culture with explants being considered viable for approximately 10 days14.
Protocol
This protocol conforms to the AVMA Animal Welfare Principles for the care and use of laboratory animals and has been approved by the Institutional Animal Care and Use Committee (IACUC) at Midwestern University.
1. Preparation of Anesthesia, Equipment, & Media
- Dechlorinate approximately 1.5 L of tap water using either a commercial dechlorinating agent (available at pet stores) or by letting water stand for 24 hr. Obtain three 1 L beakers and fill each with 500 ml dechlorinated water. Label one for holding fish before any procedure and one for recovery. To the third beaker, add 100 µg/ml of tricaine methanesulfonate; this becomes the beaker in which fish will be anesthetized.
- Fill a shallow tray with ice, warm the culture medium (RPMI 1640, 10% FBS (fetal bovine serum), 50 µg/ml gentamycin, 100 µg/ml kanamycin, 25 mM HEPES) to RT, and gather clean jeweler’s tweezers. Clean tweezers by immersing the tips in 70% ethanol and pass through a Bunsen burner, allowing the ethanol to burn off.
- Decide if the procedure will be performed without magnification, with a commercial, table-top lighted magnifying glass with 2 – 10X magnification, or dissecting microscope (entirely dependent on personal preference).
2. Establish Explant Cultures
- Catch the desired number of fish and place in a holding beaker. Transfer a single fish to the anesthesia beaker; it is best to anesthetize fish individually.
- Monitor the effects of anesthesia by observing the swimming and gill movement of the fish. As anesthesia progresses, gill movement slows and the fish will swim erratically and frequently upside down; consider fish anesthetized as soon as gill movement ceases. Do not leave the fish for longer periods in the anesthesia bath as this may result in the death of the fish.
- Remove fish from the anesthesia beaker and transfer to ice, placing the fish horizontally with the tail facing the hand holding the tweezers. If experienced with the technique and if multiple fish are to be used to make explants, consider placing the next fish in the anesthesia beaker at this time.
- To remove scales, position tweezers parallel to the fish with tip at edge of scale. Depress flat side of the tweezers slightly into the side of the fish to cause the scale to elevate from the body of the fish. Grasp scale with tweezers, pluck, and place (without inversion) in tissue culture dish. Repeat the procedure, removing a maximum of 12 scales from a large adult (6 from each side), avoiding the gill region, the lateral line, and fins.
- Place the fish into the recovery beaker and monitor to ensure it recovers from the effects of anesthesia. At this time, fish can be transferred back to an individual tank. Allow the fish to recover for at least 21 days to allow for complete scale regeneration.
- Depending on the experimental design, place up to 20 scales per plastic 35 mm tissue culture-treated dish or 4 – 6 scales per glass bottom dish. Allow scales to adhere to the dish before gently adding media so as not to detach scales from the dish.
NOTE: With experience, multiple fish can be processed before media is added to the first dish. - Add 1.2 ml of complete media (see 1.2 above) into each 35 mm dish.
- Preferably incubate explant cultures at 28 °C in 5% CO2 for the desired period of time with or without additional treatment. Alternatively, incubate cultures on a heated microscope stage or within a live cell culture chamber for video microscopy.
3. Brightfield and Video Microscopy
- Position dishes containing cell sheets onto the microscope stage and initially focus using the 4X objective lens.
NOTE: Higher magnifications may be used, depending on the experiment. Marking the location of each cell sheet and/or orientation of the dish on the stage will facilitate taking images over time with the sheets in approximately the same orientation. - Remove dishes from the incubator and photograph at various time points, according to each experimental protocol and place back into the incubator if only still images are needed over the experimental time frame.
- While performing video microscopy, pay attention to the following:
- Position the scale/emerging cell sheet within the field of view such that the migrating cell sheet remains in the field of view for the duration of the video.
NOTE: This may take some experience in observing how cells migrate from under the scale. For short videos, this is less of a concern than for longer videos. - For shorter time frames, incubate at RT. For longer time frames (18+ hr), use a heated stage or an incubation chamber to maintain temperature, pH balance, and to minimize evaporation of the culture medium.
- Position the scale/emerging cell sheet within the field of view such that the migrating cell sheet remains in the field of view for the duration of the video.
4. Fixation for Immunofluorescence Microscopy
- Prepare and pre-warm all solutions to 28 °C. In order to view keratocytes under fluorescence, grow explant cultures as described above using glass-bottom 35 mm tissue culture dishes. Alternatively, use glass chamber slides.
- Add 0.8 ml of 4% paraformaldehyde in 1.1x PBS to each dish. Do not remove the culture media first. Incubate at RT for 5 min then aspirate to remove the solution.
- Add 0.8 ml of 4% paraformaldehyde in 1.1x PBS to each dish. Incubate at RT for 5 min then aspirate to remove the solution.
- Unless using external ligands or external epitopes, permeabilize cells by adding 0.5 ml of 0.2% Triton X-100 in 1X PBS. Incubate at RT for 5 min then aspirate to remove the solution.
- Add 0.5 ml of 1% BSA in 1x PBS and incubate for 1 hr (at RT) or O/N (at 4 °C).
NOTE: Depending on experimental conditions, phalloidin can be added at this step. - After incubation, aspirate to remove the solution and proceed with antibody staining. Alternatively, store the culture dishes by adding at least 1 – 2 ml of 1% BSA in 1x PBS for 3 – 4 weeks at 4 °C.
5. Immunofluorescence Protocol
- Prepare primary and secondary antibody solutions according to the manufacturer’s recommendations.
NOTE: If manufacturer recommendations are not available, a good starting dilution of primary antibodies (in 1% BSA in 1x PBS) is 1:500 for polyclonal and 1:1,000 for monoclonal antibodies; a good starting dilution for secondary antibodies is 1:1,000. Adjust these dilutions of the original stock obtained from the manufacturer higher if the signal is weak, lower if the signal is strong and background is a problem. - Aspirate the 1% BSA in 1x PBS solution. Add 0.5 ml of primary antibody solution and incubate for 1 hr (at RT) or O/N (at 4 °C).
- Remove the primary antibody solution, place into a clean and labeled 1.5 ml microcentrifuge tube, and freeze at -20 °C.
NOTE: This allows for the reuse of the primary antibody, if needed. - Wash the dish 3 – 5 times with 1x PBS, removing and discarding the PBS after each wash.
- Add 0.5 ml of secondary antibody solution. If desired, add fluorescently-labeled phalloidin (following any manufacturer’s recommended concentration) during this step. Incubate for 1 hr (at RT) or O/N (at 4 °C).
NOTE: Use of fluorescently-labeled phalloidin allows for visualization of actin filaments; we have found that 488-labeled phalloidin works best in our zebrafish keratocytes, but other fluorophores can be used. - Remove and discard the secondary antibody solution.
- Wash the dish 3 – 5 times with 1x PBS, removing and discarding the PBS after each wash.
NOTE: The dish can be stored, covered from light, at 4 °C if unable to view immediately; make sure 1x PBS is added into the dish before storing to ensure the cells do not dry out but warm to RT (20 – 30 min), then remove the PBS just prior to proceeding to the next step. - When ready to observe, add 2 – 3 drops of a mounting medium, with or without DAPI, depending on the experimental needs, into the dish and let sit for 10 min at RT.
NOTE: We use a commercially-available, glycerol-based mounting medium with DAPI, but other mounting media can be used. Allowing 10 min for the mounting medium to sit in the dish facilitates easy removal of the glass coverslip from the bottom of the dish. - After incubation, gently squeeze on the sides of the dish to loosen and remove the coverslip. Add an additional drop of mounting media onto a glass slide and place the coverslip, cells down, onto the slide.
NOTE: Consider sealing the coverslip to the slide using clear fingernail polish if the slide needs to be kept for longer than 1 – 2 days. - Observe the slide under a fluorescence microscope.
NOTE: Most of our images were taken using a 40X or 63X objective lens under oil immersion, but the specific objective lens used will depend on the experimental protocol.
Representative Results
As illustrated in Figure 1, sheets of keratocytes can be observed migrating out from beneath the scale within hours of establishing the explant culture. Occasionally, an individually migrating keratocyte which has broken away from the collectively migrating sheet can be observed. In addition, as the explant was established from a fish which had been previously plucked, there are abundant neutrophils migrating through the sheet. At longer culture periods, the keratocyte sheet fragments as cells undergo EMT14.
The initial rate of sheet advancement is extremely rapid but this rate quickly slows as cells spread (measured by area and internuclear distance which increase ~1.8 and ~3.1 fold respectively) during the first 48 hr of culture (Figure 2A-C). The rapid increase is not associated with cell proliferation; after labeling for 24 hr with the fluorescent thymidine analog ethynyl deoxyuridine (EdU), ~10% of cells show evidence of cell division, a rate far lower than seen in transformed cell lines but more consistent with the cells involved in reepithelization in human organotypic cultures30. At later times, the sheet fragments (a phenomenon associated with the EMT observed in this system14) which makes the advance of the leading edge difficult to measure.
The area covered by sheets after 24 hr, can be used as a rapid screen for the ability of compounds to alter collective cell migration15,31. When treatment is added at the time explants are established, a dose-response in sheet area can be seen. Some mammalian cytokines (e.g., TGF-115), chemokines (such as CXCL12) and small molecule inhibitors originally characterized in mammalian systems (e.g., MMP-2, -9,and -13 inhibitors31) have been successfully employed. However, as sheet areas at 24 hr are not normally distributed (Figure 3A), non-parametric statistical tests such as a Kruskal-Wallis ANOVA must be used to determine statistical significance. For graphical display, the median standard errors of the median (upper reference limit (URL) and lower reference limit (LRL)) are plotted as these accurately describe the variation in the data. The URL and LRL are the data points (where n = number of observations) above and below the median in a rank order list of the data. When using custom error bars in Microsoft Excel, the difference between the median and each reference limit must be used (UEB and LEB in Figure 3C).
In many cases, effects of treatment(s) on collective migration are observed in shorter periods of time. In these cases, video microscopy can be used to assay response of the collectively migrating sheet to treatment. When soluble RGD containing peptide is added to the culture medium (third panel in Figure 4) to disrupt adhesion formation, the lamellae at the leading edge of the sheet rapidly shrink and subsequently the leading edge of the sheet detaches and retracts. As the entire sheet retracts in less than 2 min, response of the sheet to treatment can be rapid.
To assess localization of proteins within the sheet or within leader and follower cells, the entire sheet can be fixed and stained, as shown in Figure 5. Care must be taken during the fixation process as the cell sheets are easily disrupted. In our hands, many polyclonal antibodies to the functional domains of mammalian proteins (particularly of cytosolic proteins) have been found to cross react in our zebrafish explants.
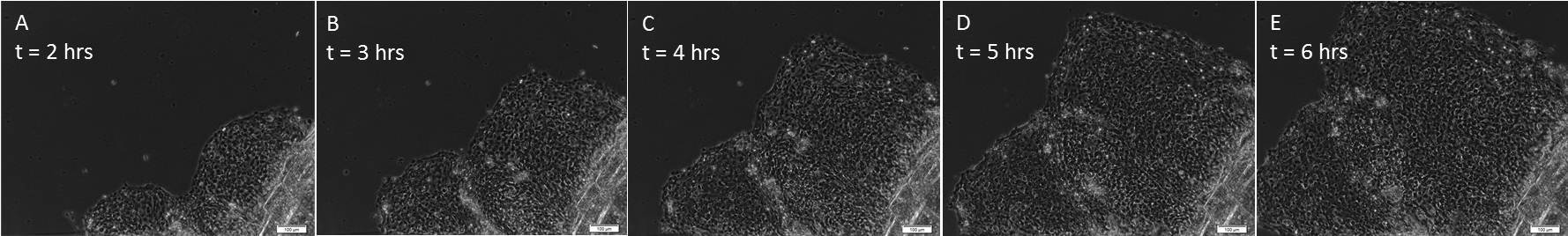
Figure 1. Collective migration of cell sheets. After establishment in explant culture, keratocytes migrate out from underneath the scale as a collective unit. (A) illustrates the relative amount of cells that have migrated away from the scale after only 2 hr in culture, with (B-E) taken each hour thereafter (3 – 6 hr, respectively). All images were taken at 100X magnification. The entire sequence is shown in Video 1; one frame was taken every 30 sec over the 6 hr time period. Please click here to view a larger version of this figure.
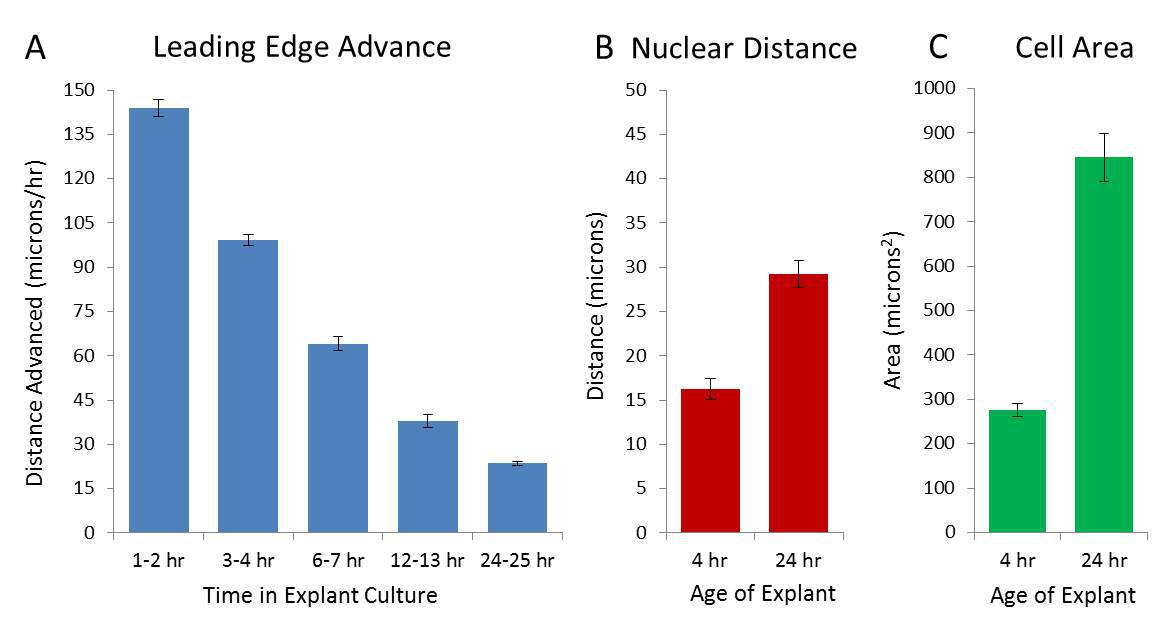
Figure 2. Characteristics of Collective Migration. Initial rate of advance of the leading edge, measured at several points, is rapid but slows down during the first 24 hr in culture (A). Measurement of internuclear distance and cell area using cultures fixed at 4 and 24 hr and stained with DAPI and phalloidin as reference indicate that during the same time period, both the internuclear distance (measured from the center of each nucleus) and the cell area increases (measured as area enclosed by the cortical actin cytoskeleton, (B) and (C) respectively). Each graph represents the mean (± SEM) of three independent experiments in triplicate. This figure has been modified from Rapanan et al.7 Please click here to view a larger version of this figure.
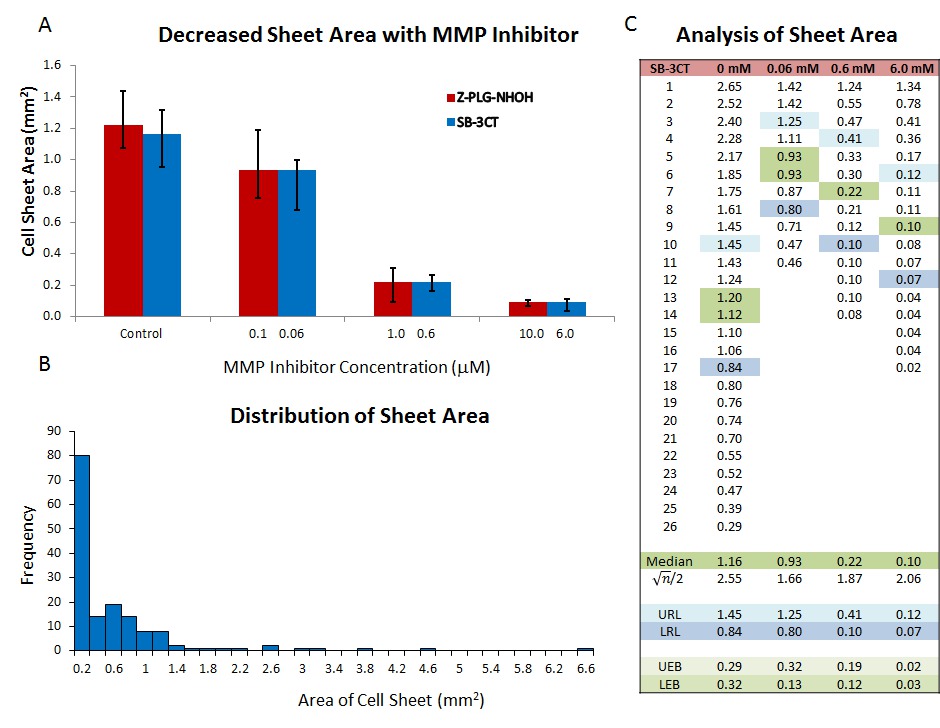
Figure 3. Assay of Collective Migration using Cell Sheet Area. When added to the culture medium during establishment of the culture, peptide Z-PLG-NHOH (broad spectrum MMP) and small molecule SB-3CT (MMP2 & 9 specific) inhibitors decrease cell sheet area after 24 hr in culture (A). As the area of untreated cell sheets is exponentially distributed (B), data should be plotted as medians with standard error of the median determined as described in the text and indicated in blue (upper reference limit or URL and lower reference limit or LRL; (C)). The difference between the median and the URL and LRL determine the size of the error bars on the graph (shaded in light green). This figure has been modified from McDonald et al.31 Please click here to view a larger version of this figure.
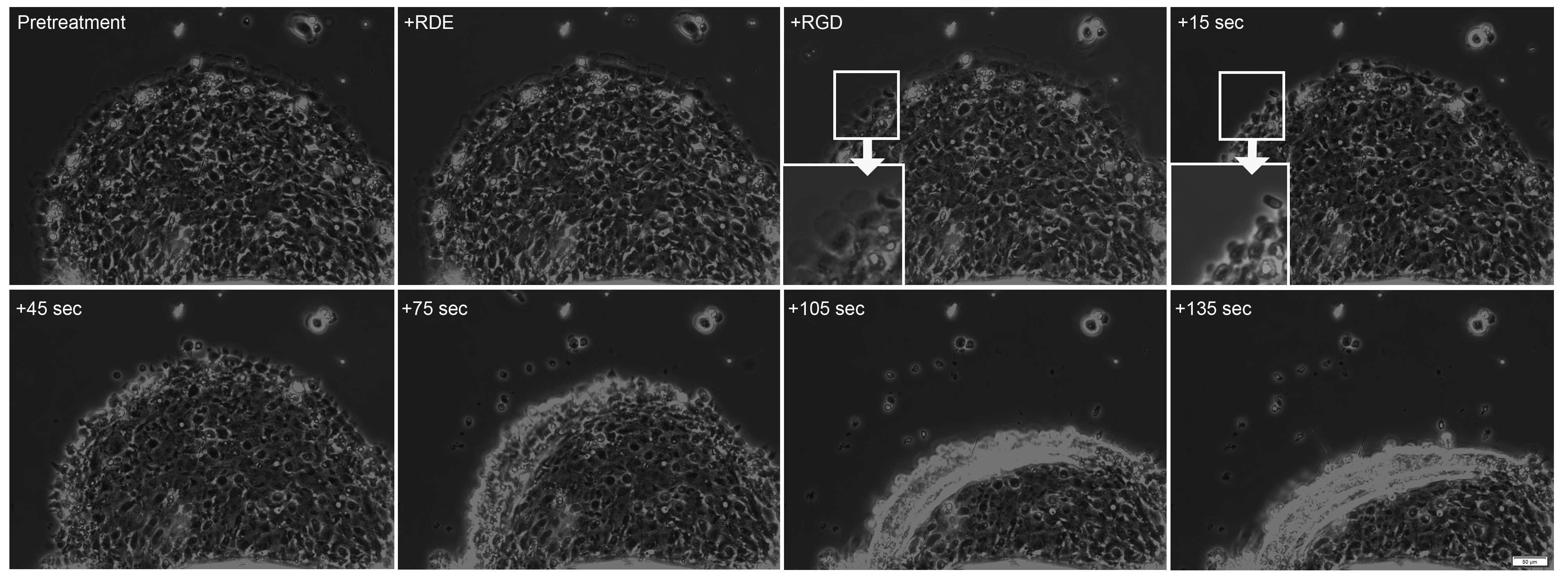
Figure 4. Addition of Soluble RGD Peptide Leads to Sheet Retraction. During pretreatment and after addition of an RGE-containing peptide, the cell sheet continues to advance. 15 sec after addition of an RGD-containing peptide, there is a decrease in the lamellae at the leading edge (inserts). After an additional 30 sec, the sheet begins to retract, a retraction which continues during the duration of the video (total video length ~5 min, see Video 2). Please click here to view a larger version of this figure.
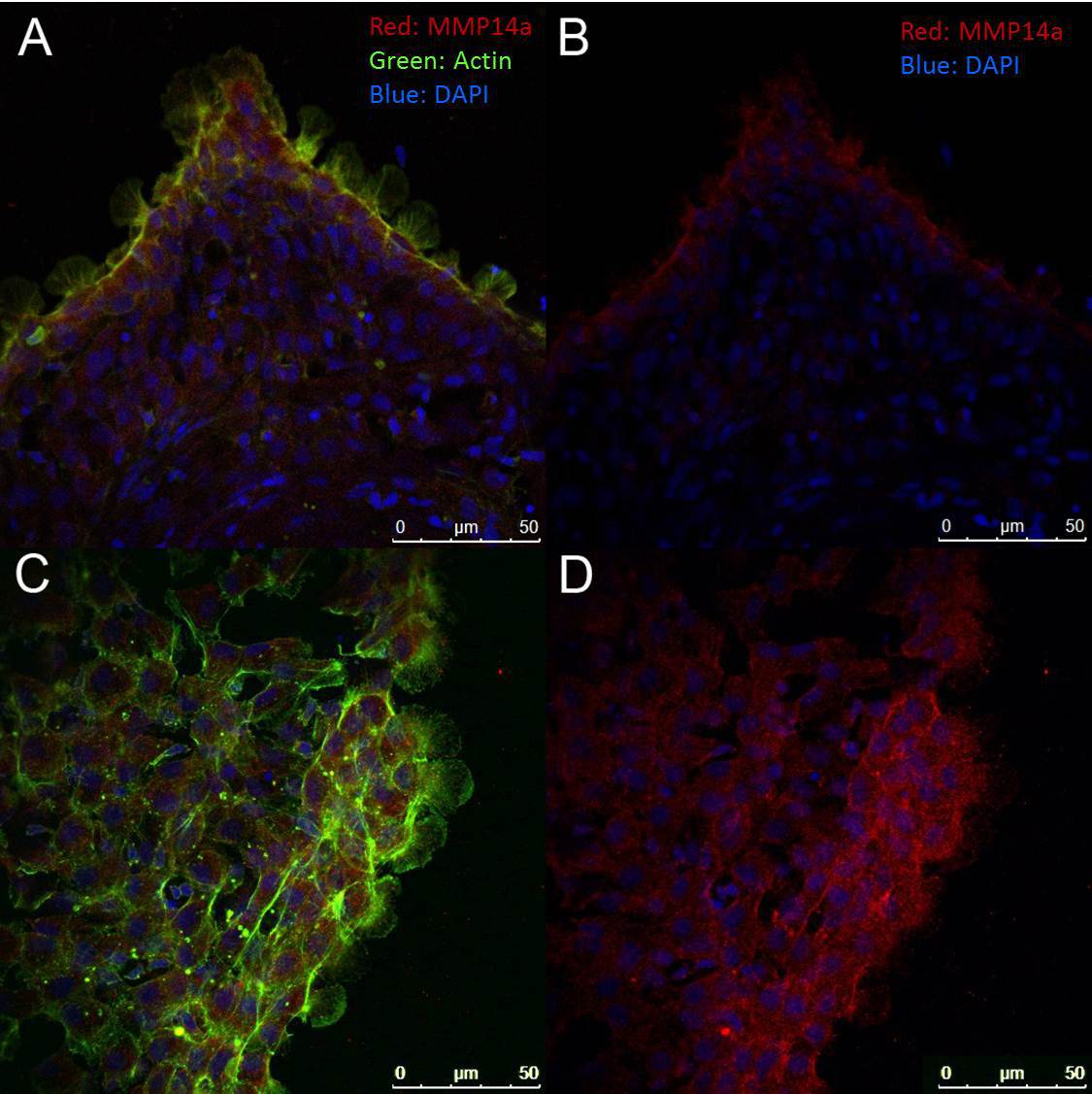
Figure 5. Immunofluorescence Assay. Cells were stained with an antibody to the catalytic domain of MMP14a (red) and counterstained with fluorescently-labeled (488) phalloidin (green) and/or DAPI (blue). (A) and (B) represent a typical 4 hr sheet emerging from the scale while (C) and (D) show a portion of a typical 24 hr sheet. All images were taken at 400X magnification. Note that the intensity of MMP14a staining is higher at the leading edge of the emerging cell sheets (seen in (B) and (D)) and prominent lamellipodia are seen in the leader cells (A) and (C)). Please click here to view a larger version of this figure.
Discussion
The most critical step for success of keratocyte explant culture is allowing the scale to adhere to the culture dish for approximately 2 – 3 min before addition of the culture medium. Approximately 75% of scales will adhere and grow sheets (the number of cultures established for each experiment will need to be adjusted accordingly). Keratocyte sheets do not form around every scale removed from the fish and placed in culture for several reasons. First, DAPI staining of explants reveals that some scales have few cells attached and thus it is expected that these scales will not have sheets of keratocytes. Second, the ability of explants to adhere to the tissue culture dish is an essential prerequisite for sheet migration. A key determinant of whether scales adhere and a successful explant culture is established is the time between placing the scale on the growth surface and the addition of media. Insufficient time to adhere without media will lead to scales floating in media (sometimes with visible tissue attached) while too long a period will result in adherent scales with no growth (presumably due to drying out of the explant with cell remnants visible at the edge of the sale). The time between scale placement in the dish and the addition of growth medium may need to be determined experimentally, as factors such as temperature and humidity within the lab room may influence how fast cells can adhere to the dish without drying out.
Although the number of commercially available, zebrafish specific antibodies is increasing, the variety of available reagents can be limiting. An 85% homology between immunogen and target protein is typically deemed necessary for cross-reactivity. However, as functional domains such as active sites, protein-protein interaction sites, and sites of modification are more conserved than other regions of the protein, anti-peptide polyclonal antibodies directed to these regions can frequently be used even if overall homology is low. For example, although zebrafish MMP14a has an overall 68% identity with its human ortholog (expect value = 0), the fraction of identity increases to 79 – 96% in catalytic and protein binding sites while cut sites are similarly highly conserved31. The data suggest that an antibody directed to the catalytically active site of human MMP14a cross-reacts with zebrafish keratocytes cultures. The suggestion that the staining in zebrafish sheets may localize to the stretched cells at the leading edge31 is consistent with other data suggesting that MMP14 may serve as a mechanical sensor32.
It is not possible to create an entirely sterile explant from a fish swimming in unsterile aquaria water. As most of our experiments are completed within 24 hr, we experience no issues with bacterial or fungal contamination of explants. However, when longer incubation periods are desired, maintaining sterility of explants may become an issue. When planning an experiment involving 3 or more days of incubation, several precautions are taken to increase the likelihood that the cultures remain uncontaminated. First, to reduce the bacterial load, the fish is allowed to swim for about 1 hr in 3 changes of fresh, dechlorinated tap water with or without the addition of 100 µg/ml kanamycin. Second, the media is exchanged on a daily basis to maintain adequate levels of antibiotic and reduce the number of any bacteria present. For particularly long incubations (>5 days) three washes with 1x PBS are performed with each media exchange. However, this introduces a variable which must be considered in the experimental design when the explant culture is treated with an exogenous compound as this will need to be replaced with the media. In addition, cytokines and growth factors secreted by the explant will be removed with each media exchange. However, as the cell number is low and the volume of media is large in a typical keratocyte explant, this effect may not be substantial.
As fish scales require 3 weeks to fully regenerate33, we recommend allowing the fish to recover for this period of time before repeated use of the fish in experiments. Although reforming the epithelial layer occurs much more rapidly, we presume that waiting this length of time between using the same fish minimizes the chance for systemic inflammatory effects which have been reported in cutaneous wound healing in fish34.
Explants have long been used to study the behavior of epithelial cells. Explants from a wide-variety of fish species and amphibians have similar motile properties at the individual and collective cell migration levels3-6,35-37. These cells have distinctly different motile properties than mammalian explants but the overall structure and organization appear to have a high degree of similarity8,30,38 although the molecular processes of EMT and wound healing appear to be well conserved14.
We have found that this protocol allows us to study the collective migration of zebrafish keratocytes using primary cell explants that model the early stages of wound healing14. Conducting experiments using newly established primary cultures avoids issues associated with serial passage of primary cells or the use of transformed cell lines. Explants may be used to study the role of EMT in cutaneous wound healing as EMT is initiated when cultures are established. Our studies suggest that these primary explants more accurately mimic the natural behavior and migration of keratocytes within the wounded epithelial layer in vivo. This protocol provides the foundation for establishing primary cultures for use in migration assays as well as for examining changes in gene and/or protein expression in a seemingly endless array of experimental conditions. The procedures are quick, easy, inexpensive, reproducible, and suitable for use at any research institution.
In summary, this explant technique provides a rapid assay for collective cell migration in the context of a wound healing model which is undergoing EMT. The presence of several cell types in multiple layers with an orienting scale provides complexity to this ex vivo culture system.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by funds from Midwestern University College of Health Sciences Research Facilitation Grant awarded to EEH and Midwestern University Office of Research and Sponsored Programs Intramural Grant awarded to KJL.
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| 35 mm Glass Bottom Culture Dishes (Poly-D Lysine Coated) | MatTek | P35GC-1.5-14-C | 1.5 mm thickness is best for coverslip removal. 14 mm diameter provides more culture area. |
| Swiss Jewelers Style Forceps, Integra, Miltex 17-303 | VWR | 21909-460 | We find that narrow, fine forceps work the best for scale removal. |
Referencias
- Burton, K., Park, J. H., Taylor, D. L. Keratocytes generate traction forces in two phases. Molecular Biology of the Cell. 10, 3745-3769 (1999).
- Keren, K., et al. Mechanism of shape determination in motile cells. Nature. 453, 475-480 (2008).
- Lacayo, C. I., et al. Emergence of large-scale cell morphology and movement from local actin filament growth dynamics. PLOS Biology. 5, 233 (2007).
- Lee, J., Ishihara, A., Theriot, J. A., Jacobson, K. Principles of locomotion for simple-shaped cells. Nature. 362, 167-171 (1993).
- Kolega, J. The Movement of Cell Clusters. J. Cell Sci. 49, 15-32 (1981).
- Kolega, J. Effects of mechanical tension on protrusive activity and microfilament and intermediate filament organization in an epidermal epithelium moving in culture. The Journal of Cell Biology. 102, 1400-1411 (1986).
- Rapanan, J. L., Cooper, K. E., Leyva, K. J., Hull, E. E. Collective cell migration of primary zebrafish keratocytes. Experimental Cell Research. 326, 155-165 (2014).
- Richardson, R., et al. Adult Zebrafish as a Model System for Cutaneous Wound-Healing Research. Journal of Investigative Dermatology. 133, 1655-1665 (2013).
- Cheon, H., et al. Increased expression of pro-inflammatory cytokines and metalloproteinase-1 by TGF-b1 in synovial fibroblasts from rheumatoid arthritis and normal individuals. Clinica., & Experimental Immunology. 127, 547-552 (2002).
- Sandeman, S. R., Faragher, R. G. A., Allen, M. C. A., Liu, C., Lloyd, A. W. Does MMP-2 expression and secretion change with increasing serial passage of keratocytes in culture. Mechanisms of Ageing and Development. 122, 157-167 (2001).
- Schnaper, H. W., et al. Increased expression of extracellular matrix proteins and decreased expression of matrix proteases after serial passage of glomerular mesangial cells. Journal of Cell Science. 109, 2521-2528 (1996).
- Tondreau, T., et al. In vitro study of matrix metalloproteinase/tissue inhibitor of metalloproteinase production by mesenchymal stromal cells in response to inflammatory cytokines: the role of their migration in injured tissues. Cytotherapy. 11, 559-569 (2009).
- Zimmermann, T., et al. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture – primary culture cells markedly differ from fourth-passage cells. Arthritis Research. 3, 72-76 (2001).
- McDonald, T. M., et al. Zebrafish keratocyte explant cultures as a wound healing model system: differential gene expressio., & morphological changes support epithelial–mesenchymal transition. Experimental Cell Research. 319, 1815-1827 (2013).
- Tan, B., Pascual, A., de Beus, A., Cooper, K., Hull, E. TGFb (transforming growth factor b) and keratocyte motility in 24-hour zebrafish explant cultures. Cell Biology International. 35, 1131-1139 (2011).
- Barut, B. A., Zon, L. I. Realizing the potential of zebrafish as a model for human disease. Physiological Genomics. 2, 49-51 (2000).
- Lieschke, G. J., Currie, P. D. Animal models of human disease: zebrafish swim into view. Nature Reviews Genetics. 8, 353-367 (2007).
- Santoriello, C., Zon, L. I. Hooked! Modeling human disease in zebrafish. The Journal of Clinical Investigation. 122, 2337-2343 (2012).
- Etchin, J., Kanki, J. P., Look, A. T., Westerfield, M., Detric, H. W., Zon, L. I. . Methods in Cell Biology. 105, 309-337 (2011).
- Li, P., White, R. M., Zon, L. I., Westerfield, M., White, H. W., Zon, L. I. . Methods in Cell Biology. 105, 403-417 (2011).
- Yen, J., et al. The genetic heterogeneity and mutational burden of engineered melanomas in zebrafish models. Genome Biology. 14, R113 (2013).
- Bouzaffour, M., Dufourcq, P., Lecaudey, V., Haas, P., Vriz, S. Fgf and Sdf-1 Pathways Interact during Zebrafish Fin Regeneration. PLoS One. 4, e5824 (2009).
- Dufourcq, P., Vriz, S. The chemokine SDF-1 regulates blastema formation during zebrafish fin regeneration. Development Genes and Evolution. 216, 635-639 (2006).
- Lee, Y., Grill, S., Sanchez, A., Murphy-Ryan, M., Poss, K. D. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 132, 5173-5183 (2005).
- Hyde, D. R., Godwin, A. R., Thummel, R. In vivo Electroporation of Morpholinos into the Regenerating Adult Zebrafish Tail Fin. Journal of Visualized Experiments. 61, e3632 (2012).
- Kizil, C., Iltzsche, A., Kaslin, J., Brand, M. Micromanipulation of Gene Expression in the Adult Zebrafish Brain Using Cerebroventricular Microinjection of Morpholino Oligonucleotides. Journal of Visualized Experiments. 75, e50415 (2013).
- Konantz, J., Antos, C. L. Reverse Genetic Morpholino Approach Using Cardiac Ventricular Injection to Transfect Multiple Difficult-to-target Tissues in the Zebrafish Larva. Journal of Visualized Experiments. 88, e51595 (2014).
- Rambabu, K. M., Rao, S. H., Rao, N. M. Efficient expression of transgenes in adult zebrafish by electroporation. BMC Biotechnology. 5, 29 (2005).
- Rapanan, J. L., Cooper, K. E., Leyva, K. J., Hull, E. E. During collective migration, leader keratocytes generate tension in sheet and may become follower or individually migrating cells. Experimental Cell Research. In press, (2014).
- Safferling, K., et al. Wound healing revised: A novel reepithelialization mechanism revealed by in vitro and in silico models). The Journal of Cell Biology. 203, 691-709 (2013).
- McDonald, T. M., et al. Matrix metalloproteinases and collective cell migration in 24 primary zebrafish explant cultures: MMP13 plays an inhibitory role and MMP14 may respond to stretch during reepithelialisation. Cell Biology International Reports. 20, 24-36 (2013).
- Haage, A., Nam, D. H., Ge, X., Schneider, I. C. Matrix metalloproteinase-14 is a mechanically regulated activator of secreted MMPs and invasion. Biochemical and Biophysical Research Communications. , (2014).
- Sire, J. -. Y., Girondot, M., Babiar, O. Marking zebrafish, Danio rerio (cyprinidae), using scale regeneration. The Journal of Experimental Zoology. 286, 297-304 (2000).
- Xu, Z., et al. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proceedings of the National Academy of Sciences. 110, 13097-13102 (2013).
- Keren, K., Yam, P. T., Kinkhabwala, A., Mogilner, A., Theriot, J. A. Intracellular fluid flow in rapidly moving cells. Nature Cell Biology. 11, 1219-1224 (2009).
- Bereiter-Hahn, J., Strohmeier, R., Kunzenbacher, I., Beck, K., Voth, M. Locomotion of Xenopus epidermis cells in primary culture. Journal of Cell Science. 52, 289-311 (1981).
- Nishikawa, A., Shimizu-Nishikawa, K., Miller, L. Isolation, characterization, and in vitro culture of larval and adult epidermal cells of the frog Xenopus laevis. In Vitro Cellula., & Developmental Biology. 26, 1128-1134 (1990).
- Schober, M., et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. The Journal of Cell Biology. 176, 667-680 (2007).

