Inducing Axonal Varicosities with a Micromechanical Stimulus on Neurons
Abstract
Source: Servello, D., et al. A Microbiomechanical System for Studying Varicosity Formation and Recovery in Central Neuron Axons. J. Vis. Exp. (2018).
This video demonstrates the induction of axonal varicosities by applying micromechanical stimulus on neurons. The system comprises a micropipette attached to a syringe containing buffer via tubing. Using a micromanipulator, the micropipette is positioned above the neurons. Fluid pressure is then applied to induce varicosities in axons.
Protocol
1. Setting Up the Puffing Pipette Apparatus
NOTE: There are five basic components to this set-up: the glass pipette, the tubing, the syringe, the micromanipulator, and the buffer (Hank's) Table 1. Any buffer that has physiologically relevant salt concentrations could be used in this set-up.
- Pull a borosilicate pipette to have around 45-µm-diameter opening tip using a micropipette puller and the specifications in Table 2.
NOTE: It was found that 45 µm is a suitable size for the diameter of the pipette opening under experimental conditions. Slight variations in the opening size should not significantly affect results. It is important to note that larger departures from this number should still work for varicosity induction by adjusting the height of the syringe that is linked to the pipette via tubing, but this will require recalibration of the pressure value. - Insert the un-pulled end of the pipette into thin rubber tubing.
NOTE: The tubing wall should be thin and rigid to minimize the fluid resistance, ensuring that the height of the syringe is directly proportional to the pressure of fluid flowing through the pipette with negligible resistance. The tubing should be no longer than about 0.6 m in length to further minimize the resistance. - Connect the other end of the tubing to a 10-mL plastic syringe through a connector with a turnable valve.
NOTE: The plastic syringe needs to be held at a constant, measurable height to ensure an accurate measure of the assumed pressure flowing from the pipette. A height of 190 mm was utilized since this provides minimal pressure but reliably induces varicosities in axons. Other height values may also be used if the pressure causes the cells to detach from the coverslip surface. It is recommended that the same setting be used throughout the experiment. - Insert the pipette into the screw top of the micromanipulator to hold the pipette in place. Screw the metal, flat-topped screw attached to the micromanipulator arm so that it holds the un-pulled end of the pipette just above where the rubber tubing is attached. Angle the pipette at a 45° angle with the surface of the neuron-containing coverslips.NOTE: The micromanipulator should be constructed so the pipette can be held without constricting the rubber tubing. The micromanipulator must allow motion of the pipette in the x, y, and z directions to accurately position it within range of the cells. A graphic and a photograph of the apparatus are provided below (Figure 1).
- Turn on a fluorescence filter, open the aperture, and ensure a fluorescent spot can be seen coming through the objective. Using the micromanipulator, move the pipette into a position that lines up the tip with the fluorescent spot and lower the pipette into a position just above the height of the cell culture dish (35 mm x 10 mm). Once it is in position, turn on the transmitted light and turn off fluorescence.
- Using tweezers, add one coverslip (with the cells facing up toward the pipette) from the cell culture plate to the cell culture dish containing about 2 mL of Hank's buffer at room temperature.
- Place the culture dish containing the coverslip onto the scope.
- Using the fine focus knob and 20X objective, focus on a plane about four full turns above the cells (about 0.4 mm). Use the micromanipulator to position the pipette tip so that it is focused and in the center, left-hand side of the plane as seen through the eye-pieces.
NOTE: Dendrites are projections that should be in the same frame as the cell body they originate from. To induce axonal varicosities, one should follow longer projections from the cell body to medial and distal axons.
2. Calibrating the Pressure of the Pipette Utilizing a Stretchable-membrane System
- Setup the puffing pipette apparatus with the exact same parameters as described above over a system containing a microfabricated silicone membrane (500 µm in diameter and 50 µm thick) and focus the microscope onto the surface of the membrane.
NOTE: This system was designed to measure small pressure values, and an explanation of the system has been published before. This measurement only needs to be done once for puffing experiments if the exact same setting of puffing is used. - Focus the microscope on the arrays of microdots (4 µm in diameter and 10 µm apart, as measured between centers). Turn the stop valve and allow fluid to flow out of the pipette. Examine the deflection of the membrane in response to the fluid pressure with a confocal microscope.
- Utilize a linear model to determine the corresponding pressure associated with the deflection of the membrane.
NOTE: While a recent study found that a deflection of w= -3.143 ± 0.69 µm was obtained for 190 mm syringe height, this produced a pressure of 0.25 ± 0.06 nN/µm2 from the current pipette setup, which is within the physiological and pathological range, though the syringe height is not the sole determinant. Other factors influence the exact pressure value on the neurons as well, including pipette opening, positioning (distance and angle) of the pipette tip relative to the cells, and even the flexibility of the tubing.
3. Puffing to Induce Varicosities
- Once the pipette is in position, focus the microscope on a plane that contains a region of interest. Position the cells and processes in the left half of the imaging field (seen by live capture of the camera) within the puffing area, while the right half is outside of the puffing area.
NOTE: Due to the extensive outgrowth of axons and dendrites of neurons, it is unlikely that all the processes of a neuron are within the same puffing area. This is actually one advantage of this system, which allows the examination of the local effect of puffing. There are two types of puffing that can be utilized to induce varicosities: long-duration puffing and pulse puffing. - Puffing
- Long duration puffing
- Position the syringe at the height, 190 mm above the coverslip containing cultured neurons. Begin time-lapse imaging of area of interest with an optimized exposure time revealing clear neuronal morphology. Capture at least 15 frames at a 2 s interval (30 s total) as the baseline. The interval can be adjusted based on the experiment. At frame 16, open the turn-able stop valve of the syringe and allow fluid to flow for 75 frames (150 s). At frame 75, turn the valve off so that fluid flow stops. Stop video capture.
NOTE: This should induce axonal varicosities in neurons 7-9 DIV within 5-10 s, whereas older neurons take longer to form varicosities.
- Position the syringe at the height, 190 mm above the coverslip containing cultured neurons. Begin time-lapse imaging of area of interest with an optimized exposure time revealing clear neuronal morphology. Capture at least 15 frames at a 2 s interval (30 s total) as the baseline. The interval can be adjusted based on the experiment. At frame 16, open the turn-able stop valve of the syringe and allow fluid to flow for 75 frames (150 s). At frame 75, turn the valve off so that fluid flow stops. Stop video capture.
- Pulse (2 s duration) puffing
- Position the syringe at the proper height (190 mm). Begin time-lapse imaging and capture at least 15 frames (30 s) at a 2 s interval. At frame 16, open the valve of the syringe and allow fluid to flow, then close the valve after 2 s. Wait 5-20 s (depending on the frequency to be tested) before opening the valve again. Repeat opening and closing of the valve to create fluid pulses, and continue for a total period of 150-300 s. Continue time-lapse imaging for 20 min to capture the recovery stage.
NOTE: When the interval is longer than 10 s, the pulses induce drastically less varicosities. At a 20 s interval, no varicosities can be induced by the pulses. To obtain reliable, consistent varicosity formation, it is advised the syringe height should be around 190 mm. Syringe height and fluid pressure should not be raised to a point where cells begin detaching from the coverslip surface.
- Position the syringe at the proper height (190 mm). Begin time-lapse imaging and capture at least 15 frames (30 s) at a 2 s interval. At frame 16, open the valve of the syringe and allow fluid to flow, then close the valve after 2 s. Wait 5-20 s (depending on the frequency to be tested) before opening the valve again. Repeat opening and closing of the valve to create fluid pulses, and continue for a total period of 150-300 s. Continue time-lapse imaging for 20 min to capture the recovery stage.
- Long duration puffing
- Before a day of use, clean the set-up (pipette, tubing, and syringe) by flowing about 10 mL 70% Ethanol through the set-up. Following the ethanol wash, flow 10 mL of Hank's buffer (or desired buffer) through the set-up to prime the system for that day's use. After finishing experiments for the day, clean the set-up as previously described with ethanol to prevent the growth of contaminants overnight.
Table 1: Hank's buffer recipe. This recipe prepares the buffer utilized in the puffing protocol and during live-cell imaging.
| Hank's buffer (filter, pH = 7.4, store at 4 °C | |
| 150 mM | NaCl |
| 4 mM | KCl |
| 1.2 mM | MgCl2 |
| 1 mM | CaCl2 |
| 10 mg/mL | D-glucose |
| 20 mM | HEPES |
Table 2: Pipette pulling parameters. Provides the parameters to be set on the pipette puller to obtain an opening that is around 45 µm in diameter.
| Pipette pulling parameters | |||||
| Heat | Pull | Velocity | Delay | Pressure | Ramp |
| 501 | 0 | 15 | 1 | 500 | 530 |
Representative Results
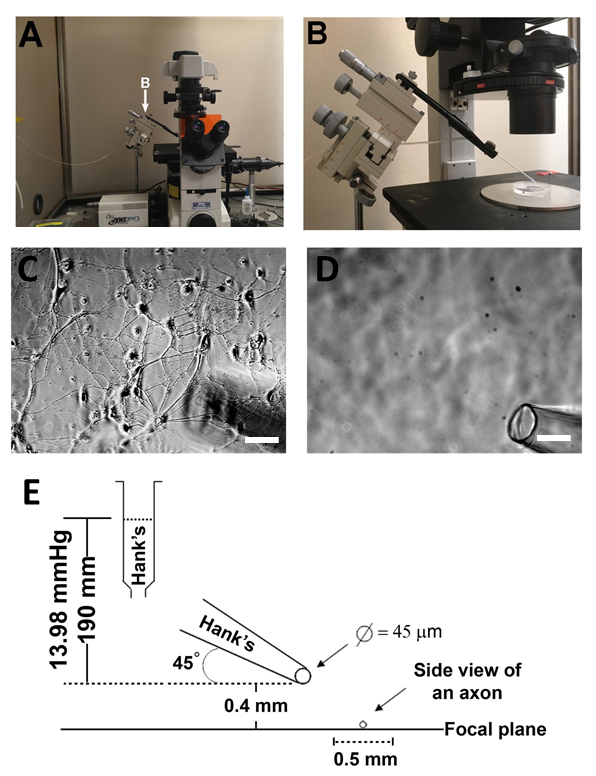
Figure 1: Schematic and photograph of puffing apparatus. (A) Photographs showing the overall microscope set-up. (B) The puffing apparatus showing the glass pipette held by the micromanipulator and connected to the rubber tubing. (C) Phase contrast images with a focus on the plane of primary hippocampal neurons showing the shadow of the pipette in the bottom right. (D) The out-of-cell plane is focused on the puffing pipette tip. (E) Schematic of distances and calculated pressures for inducing varicosities. Scale bar = 50 µm.
Divulgaciones
The authors have nothing to disclose.
Materials
| ubber tubing | Fisher Scientific | 14-169-1A | |
| 10cc plastic syringe and plunger | Becton Dickinson | ||
| micromanipulator | Sutter Instruments | ||
| Cell culture dish (35 mm x 10 mm) | Corning | 3294 | |
| Model P-1000 Flaming/Brown Micropipette puller | Sutter Instruments | ||
| Eclipse TE2000-U Mcroscope | Nikon | ||
| Plan Fluor ELWD 20x lens | Nikon | 62933 | objective |
| Apo TIRF 100x/1.49 oil lens | Nikon | MRD01991 | objective |
| HEPES | Fisher Scientific | BP410-500 | |
| D-glucose | Fisher Scientific | D16-500 | |
| MgCl2 | Fisher Scientific | BP214-500 | |
| NaCl | Fisher Scientific | S640-3 | |
| KCl | Fisher Scientific | BP366-500 | |
| CaCl2 | Fisher Scientific | C70-500 |

