Isolation and Culture of Primary Oral Keratinocytes from the Adult Mouse Palate
Summary
The present protocol describes the isolation and culture of oral keratinocytes derived from the adult mouse palate. An evaluation method using immunostaining is also reported.
Abstract
For years, most studies involving keratinocytes have been conducted using human and mouse skin epidermal keratinocytes. Recently, oral keratinocytes have attracted attention because of their unique function and characteristics. They maintain the homeostasis of the oral epithelium and serve as resources for applications in regenerative therapies. However, in vitro studies that use oral primary keratinocytes from adult mice have been limited due to the lack of an efficient and well-established culture protocol. Here, oral primary keratinocytes were isolated from the palate tissues of adult mice and cultured in a commercial low-calcium medium supplemented with a chelexed-serum. Under these conditions, keratinocytes were maintained in a proliferative or stem cell-like state, and their differentiation was inhibited even after increased passages. Marker expression analysis showed that the cultured oral keratinocytes expressed the basal cell markers p63, K14, and α6-integrin and were negative for the differentiation marker K13 and the fibroblast marker PDGFRα. This method produced viable and culturable cells suitable for downstream applications in the study of oral epithelial stem cell functions in vitro.
Introduction
The oral epithelium serves as a first barrier in protecting the body from environmental stresses, including chemical or physical damage and bacterial and viral infections1,2. The oral mucosa comprises an outer layer of stratified squamous epithelium that consists of keratinocytes and underlying connective tissue called the lamina propria, which mainly consists of fibroblasts and the extracellular matrix. The mouse oral mucosa can be broadly divided into three subtypes: masticatory (hard palate and gingiva), specialized (dorsal tongue), and lining (buccal mucosa, ventral tongue, soft palate, lips) mucosa2,3 (Figure 1A). The oral epithelium is keratinized in the masticatory and specialized mucosa and non-keratinized in the lining mucosa. Despite its anatomical location, the oral epithelium is similar to the skin epidermis in that it consists of tightly packed epithelial cells with varying degrees of differentiation: basal layers containing undifferentiated cells; spinous, granular, and cornified layers that form keratinized epithelium, or intermediate and superficial layers that form non-keratinized epithelium4. Transgenic mouse models have facilitated the study of oral epithelial stem cells' cellular and molecular features in the palate, buccal mucosa, tongue, and gingiva5,6,7,8,9,10,11. However, most of these studies primarily used in vivo mouse experiments. Cell culture systems were not typically employed owing to a lack of established and efficient protocols.
An in vitro culture system can be used for the molecular and biochemical analysis of stem cell regulators, cell-based assays, and drug screening. Currently, protocols for the culture of primary keratinocytes of the skin epidermis have been developed, in which basal keratinocytes can be successfully isolated and cultured for clinical and research purposes12,13,14,15. In 1980, Hennings et al. showed that a low calcium concentration (< 0.09 mM Ca2+) in the culture medium facilitated proliferation and maintained cells in an undifferentiated state. A higher level of calcium promoted cell differentiation and reduced proliferation16. Subsequently, culture methodologies for neonatal and adult murine epidermal keratinocytes have been established and widely applied to numerous mouse models with different genetic backgrounds for in vitro studies17,18,19. Although skin and oral epithelia share common characteristics, they also show intrinsic differences, e.g., in their keratinization status, turnover rate, gene expression, and wound healing ability3,11,20,21,22,23,24,25,26.
Although human oral keratinocyte culture has been successfully performed27,28,29, publications on mouse oral keratinocyte culture30,31,32 are limited due to the small size of the target tissue and the distinct characteristics of the cells compared to skin epidermal keratinocytes. This protocol describes the isolation and long-term culture techniques of mouse primary oral keratinocytes.
Protocol
All animal experiments were performed according to the Institutional Animal Experiment Committee guidelines at Kumamoto University and the University of Tsukuba.
1. Preparation of reagents and culture media
- Prepare 40 mL of keratinocyte culture medium containing 60 µM of calcium and 600 µL of antibiotic-antimycotic solution. Prepare 20 mL of 0.025% trypsin and 400 µL of antibiotic-antimycotic solution.
- Thaw the trypsin inhibition solution at room temperature and keep it at 4 °C until use in step 4.1.
NOTE: The isolation reagent is prepared for the tissue isolation of five mice.
- Thaw the trypsin inhibition solution at room temperature and keep it at 4 °C until use in step 4.1.
- To prepare the culture media, take 500 mL of medium and add 5 mL of a growth supplement solution (see Table of Materials) (hereafter referred to as complete medium) and 20% calcium-depleted chelexed-fetal bovine serum (FBS)33 (hereafter referred to as chelexed-FBS).
2. Dissection of palate tissue from adult mouse
- Sacrifice an adult C57BL/6J mouse (either male or female) by cervical dislocation in compliance with the facility's regulations relating to animal welfare.
- Remove the hair around the mouth with a shaver. Using scissors, cut from the cheek toward the jaw, on both sides.
NOTE: The mouse needs to be anesthetized before sacrifice. An anesthetic mixture of medetomidine, midazolam, and butorphanol is used (see Table of Materials).
- Remove the hair around the mouth with a shaver. Using scissors, cut from the cheek toward the jaw, on both sides.
- Use forceps to open the mouth wide and absorb any blood using a cotton swab. To disinfect the palate, wipe the inside of the mouth with a cotton swab containing 10% povidone-iodine.
- To harvest the mouse palate, first, use a surgical scalpel blade to make a full-thickness marginal incision along the palate side of the maxillary teeth. Then, carefully dissect the entire palate using a raspatorium.
NOTE: A raspatorium is a tool used to elevate a mucoperiosteal flap (see Table of Materials) (Figure 1B). - Quickly transfer the palate tissue to a 15 mL tube containing 4 mL of complete medium + antibiotic-antimycotic solution. Keep the tissues on ice until ready for incubation.
NOTE: For collection from multiple mice, palate tissues may be kept on ice for up to 4 h.
3. Pretreatment of palate tissue
- In a laminar flow hood, transfer tissues to a 60 mm dish containing 4 mL of complete medium + antibiotic-antimycotic solution.
- Using short, blunt forceps and a scalpel blade, gently remove any blood from the tissues. Wash tissues 10 times in complete medium.
- Transfer tissues to a 35 mm dish containing 4 mL of 0.025% trypsin + antibiotic-antimycotic solution, with the epithelial surface facing down.
NOTE: The epithelial surface, which curves inward, should be soaked in the trypsin solution; the lamina propria should face up. The tissue should be flattened as much as possible to be incubated entirely in the trypsin solution. - Incubate tissues in 0.025% trypsin for ~16 h at room temperature in the culture hood.
4. Collection and culture of primary cells
- Using one pair of blunt forceps, remove tissue from trypsin solution (in the 35 mm dish) and transfer to trypsin inhibitor solution in a 60 mm dish (4 mL per dish) with the epithelial surface facing up.
- Using forceps to hold onto the edge of the palate, gently scrape the epithelial layer off the underlying lamina propria using a scalpel blade.
NOTE: Connective tissue is not digested by trypsin, so it does not peel off during scraping. - To collect the maximum amount of epithelial cells from tissues, transfer the tissue into another 60 mm dish with 4 mL complete medium and repeat the scraping step.
NOTE: Make sure to avoid scraping the tissue with the blade's tip; use the blade's edge instead. Scraping is performed for 5-10 min per tissue. - Place a sterile 100 µm cell strainer on the top of a 50 mL conical tube.
- Using a sterile pipette, transfer 2 mL of trypsin solution (from step 4.1) into the strainer to wet its surface.
- Using a pipette, mix the cell suspension in the 60 mm dish (from steps 4.2-4.3) a few times and filter cells through the 100 µm cell strainer prepared in steps 4.4-4.5.
- Count the number of cells using a hemocytometer. Prepare 15 µL of trypan blue solution and add 15 µL of cell suspension (step 4.6). Transfer 10 µL of the cell-trypan blue mix to a hemocytometer and count the number of cells.
NOTE: One piece of mouse palate can yield up to 1 million cells. - While counting, centrifuge the tube (from step 4.6) at 100 x g for 5 min at room temperature.
- Aspirate the supernatant with a pipette. Add 2 mL of complete medium + chelexed-FBS to the tube. Resuspend the cell pellet by triturating several times using a 5 mL pipette.
- Plate 2-5 x 105 cells from one mouse into one well of a 24-well plate pre-coated with Collagen Type I (see Table of Materials).
- Incubate the cells at 37 °C for 2 days without changing the medium.
- Two days after seeding, replace half of the culture medium with the complete medium + chelexed-FBS. Check the cell morphology under the microscope. Feed cells with complete medium + chelexed-FBS every 2 days.
NOTE: Following seeding, cells of different sizes will be observed. Approximately 3-5 days after seeding, keratinocytes with a cobblestone morphology (Figure 2) can be observed. It will take 1-2 weeks of culture before the first passage can be conducted and a subsequent 1-2 weeks is necessary before the cells are ready for the second and third passages. After that, cells will grow faster and may be prepared for cryopreservation. Cells can be re-plated to one well (of a 24-well plate) and a 6-well plate in the first and second passage, respectively. The subsequent passage will be dependent on the cell growth and density. A cell split ratio of 1:2 or 1:3 can be used after the third passage.
5. Keratinocyte passage
- Collect 2 mL of the supernatant from the culture dish and dispense into a 15 mL conical tube (on ice). Wash the cells with sterile 1x PBS twice.
NOTE: When the cells reach approximately 70%-80% confluency, they are ready for passage. - Add 1 mL of 0.05% trypsin-EDTA to the dish to the one well of a 6-well plate.
- Incubate for 5-15 min at 37 °C; check after 5 min to see whether the cells detach from the dish.
- Neutralize the reaction using 1 mL of trypsin inhibition solution and 2 mL of complete culture medium + chelexed-FBS by gentle pipetting. Next, transfer the cell suspension into the same 15 mL conical tube as in step 5.1.
- Centrifuge the cell suspension at 100 x g for 5 min at 4 °C. Aspirate the supernatant with a pipette and resuspend the cell pellet in 1 mL of complete culture medium.
- Count the cells using a hemocytometer. Next, plate 1 mL of the cell suspension into a new 24- or 6-well culture plate.
NOTE: Some of the cells from the early passages can be frozen in a mixture of 70% complete culture medium + 20% chelexed-FBS + 10% DMSO. 1-2 cryovials of cells can be collected from one confluent culture plate.
6. Cryopreservation and recovery of keratinocytes
- Cell freezing
- Grow keratinocytes to 80%-90% confluency.
NOTE: Do not allow cells to overgrow, as this could reduce their proliferation status and viability. - Treat the keratinocytes in the dish with 0.05% trypsin-EDTA, as described in steps 5.1-5.5.
- Count the keratinocytes using a hemocytometer. Prepare cryovials based on calculated cell numbers to allow for the transfer of 1 x 106 cells/mL to each vial.
- Centrifuge the cell suspension at 100 x g for 5 min at 4 °C. Discard the supernatant and resuspend the cell pellet in a 10 mL solution of 10% DMSO + 20% chelexed-FBS + 70% complete culture medium (9 mL of complete medium + chelexed-FBS and 1 mL of DMSO).
- Dispense the cells into cryovials at 1 mL of suspension per vial. Place the vials in a cryogenic storage container overnight at -80 °C. Transfer the vials to a liquid nitrogen tank the following day.
- Grow keratinocytes to 80%-90% confluency.
- Cell recovery
- Remove a cryovial from the liquid nitrogen tank and partially thaw at room temperature. In a 15 mL tube, mix 1 mL of the cell suspension with 3 mL of complete culture medium + chelexed-FBS.
- Centrifuge the mixture for 5 min at 100 x g and 4 °C. Discard the supernatant and resuspend the pellet in 1 mL of complete culture medium + chelexed-FBS.
- Plate the cell suspensions into new 60 mm Collagen I-coated culture dishes. Replace the culture medium every 2-3 days and passage the cells once confluent.
7. Immunofluorescent staining
- Culture oral keratinocytes on square coverslips (22 mm x 22 mm) in 6-well plates (5 x 105 cells/well) for 2 days.
- Fix keratinocytes in a solution of 4% paraformaldehyde (PFA) and PBS for 20 min at room temperature before washing three times with 1x PBS.
- Permeabilize cells in a solution of 0.1% Triton in PBS. Incubate cells in blocking reagent (2.5% goat serum, 2.5% donkey serum) for 1 h at room temperature, followed by overnight incubation with primary antibodies (see Table of Materials) at 4 °C.
NOTE: Primary antibodies were used at the following dilutions: rabbit anti-K14 (1:1000), rat α6-integrin (1:100), rabbit anti-p63 (1:500), rabbit anti-K13 (1:100), and goat anti-PDGFRα (1:100). - Wash samples in a solution of 0.1% Triton in PBS, followed by incubation with secondary antibodies for 1 h at room temperature.
NOTE: The secondary antibodies (Alexa 488 or 555) were used at a 1:300 dilution. - Counterstain all samples with Hoechst solution for 10 min and mount cells onto glass slides.
NOTE: Hoechst solution is used to stain the nuclei of cells. - Perform sample imaging using a confocal microscope.
NOTE: The brightness and contrast are adjusted to equal intensity using image editing software.
Representative Results
Overview of the dissection process and isolation of oral keratinocytes from the adult mouse palate
Dissociated oral keratinocytes were collected from the adult mouse palate and cultured in a customized 20% chelexed-FBS. The mouse palate consists of the hard palate and the soft palate (Figure 1C). The procedure for the isolation of mouse oral keratinocytes is summarized in Figure 1D. The palate tissue is dissected and transferred to a media containing an antibiotic-antimycotic solution before being incubated in 0.025% trypsin solution at 4 °C overnight. The following day, the palate tissue is treated with trypsin inhibitor solution and complete culture medium in equal volumes. Subsequently, the tissues are scraped using a surgical scalpel blade to collect oral keratinocytes. The cell suspension is filtered through a 100 µm cell strainer and centrifuged. The cells are then seeded in Collagen I-coated 24-well plates containing 2 mL of complete culture medium + chelexed-FBS.
Representative results of the successful isolation of mouse oral keratinocytes
Primary oral keratinocytes grew as a monolayer and displayed a cobblestone morphology (Figure 2). Small keratinocyte colonies were visible at 3-5 days (Figure 2A,B); these grew larger and formed tight colonies at 1 week of incubation (Figure 2C). Keratinocyte colonies displayed the typical morphological features of basal keratinocytes, indicating their healthy conditions. Human oral keratinocytes remained undifferentiated for several passages in the complete culture medium containing 0.06 mM Ca2+16,28. The first passage was performed approximately 2 weeks from the initial plating (Figure 2D). At later passages, keratinocytes exhibited stable growth with a shorter period of culture (Figure 2E–G). Keratinocytes stopped growing if significant fibroblasts contamination occurred during the isolation process (Figure 2H).
Isolated mouse oral keratinocytes express basal cell markers
To confirm the status of primary oral keratinocytes, immunostaining was performed using the basal cell markers Keratin 14 (K14) and α6-integrin34. K14 and α6-integrin were expressed in keratinocytes after culturing (Figure 3A,B). The cells were also stained with stem cell marker p63 to confirm their stemness. Early passage (passage 4) and late passage (passage 7) cells showed uniform expression of p63 (Figure 3C,D). In contrast, keratinocytes treated with high calcium (1.2 mM induction for 2 days) exhibited decreased p63 expression (Figure 3E), indicating that high calcium treatment suppresses stem cell-related genes in primary keratinocytes as previously reported16. The differentiation marker Keratin 13 (K13) showed rare or no expression in both early and late passages and significant expression under high calcium treatment (Figure 3F–H). To test the possibility of fibroblast contamination in the keratinocyte culture, staining using the fibroblast marker PDGFRα was performed with the same set of keratinocytes compared with mouse embryonic fibroblast (MEFs). There was no expression of PDGFRα in the keratinocyte culture, compared with the high expression observed in MEF cells (Figure 3I–3L). These results indicated that this protocol could successfully isolate basal keratinocytes and maintain these cells in the undifferentiated state.
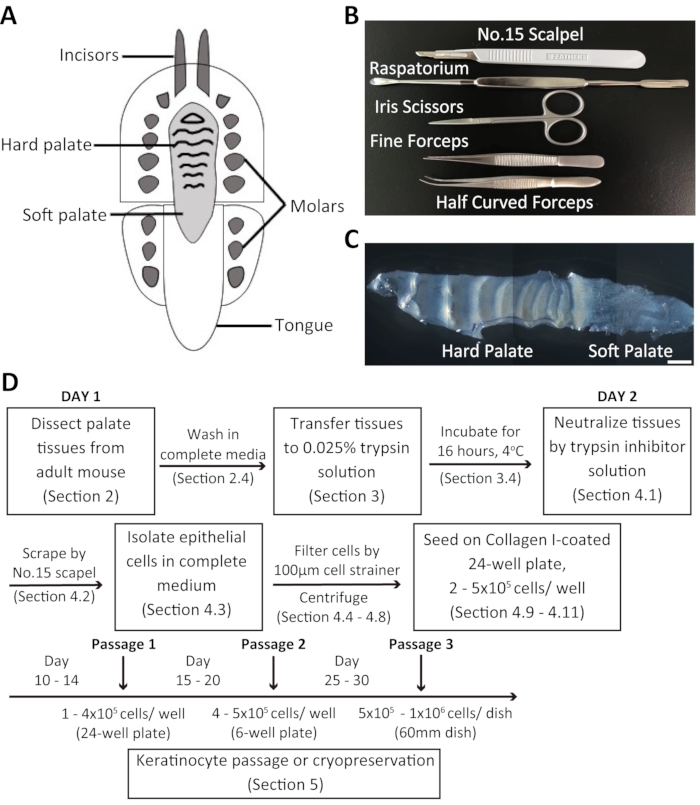
Figure 1: Overview of the dissection procedure and isolation of mouse oral keratinocytes. (A) Schematic representation of the mouse oral cavity. (B) Instruments used to dissect the palate and isolate mouse oral keratinocytes. (C) Brightfield image of the mouse palate. Scale bar: 100 µm. (D) Summary of the protocol. Please click here to view a larger version of this figure.
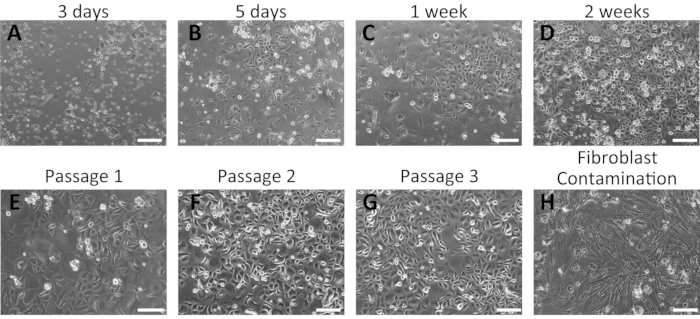
Figure 2: Representative results of the successful isolation of mouse oral keratinocytes. (A–G) Time-course images of cultured primary oral keratinocytes at 3 days (A), 5 days (B), 1 week (C), and 2 weeks (D) of culture after isolation. Morphologies of mouse oral keratinocytes after the first (E), second (F), and third (G) passages are shown. (H) Example of fibroblast contamination in mouse oral keratinocyte culture. Scale bar: 400 µm. Please click here to view a larger version of this figure.
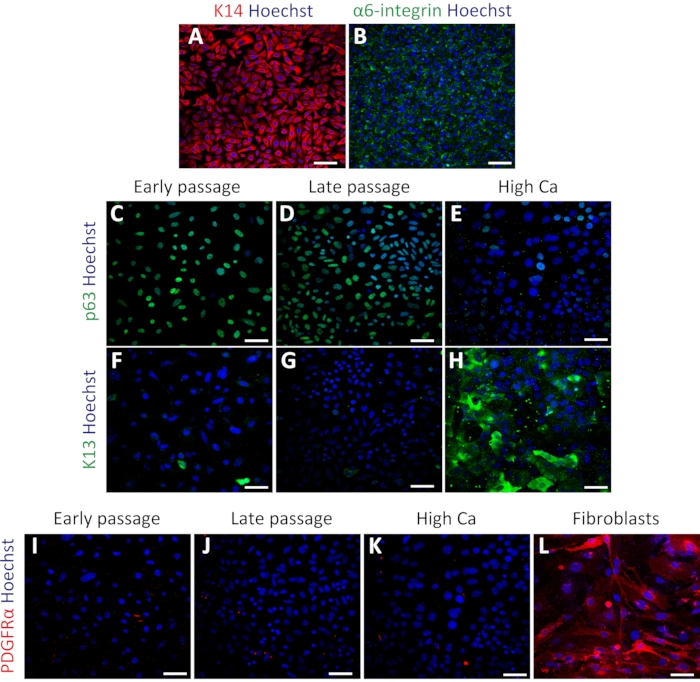
Figure 3: Isolated mouse oral keratinocytes express basal cell markers. (A–B) Representative images of immunofluorescent staining of K14 (A; red) and α6-integrin (B; green) in passage 4. (C–E) Representative images of immunofluorescent staining of p63 (green) in passage 4 (C), passage 7 (D), and high calcium treatment (E). (F–H) Immunostaining images of K13 (green) in passage 4 (F), passage 7 (G), and high calcium treatment (H). (I–L) Immunostaining images of PDGFRα (red) in passage 4 (I), passage 7 (J), high calcium treatment (K), and MEFs (L). Nuclei are stained with Hoechst (blue). Scale bars: 100 µm. Please click here to view a larger version of this figure.
Discussion
Primary keratinocytes isolated from human or mouse skin epidermis have been utilized for many years in research and clinical applications12,13,15,18,27,28,29. By contrast, few protocols have been established to isolate and culture primary oral keratinocytes from adult mice30,31,32. The present study used a commercial complete culture medium and chelexed-FBS to maintain keratinocytes in a proliferative or stem cell-like state. This culture system can be employed in molecular and biochemical assays to further understand the features of oral epithelial stem cells and their related diseases.
Several critical steps are included in this protocol. Firstly, the trypsin concentration and the incubation time are essential in producing viable cells for subsequent cultures. We consistently used 0.025% trypsin solutions and 16 h incubation periods in the chamber hood at room temperature. If not incubated for a sufficient length of time, keratinocytes would not properly dissociate from the tissue, resulting in a lower final cell yield. Secondly, gentle pipetting of the cell suspension on the second day notably affects cell viability. Scraping should gently start from the epithelial side and not exceed 10 min per tissue sample. Finally, the first isolated cell suspension contains fibroblasts and other cell types; these unwanted cells will usually begin to disappear in subsequent cultures.
Potential limitations were identified during cell isolation and culture. In rare cases, fibroblasts may be contaminated during the isolation process, and the fibroblasts may inhibit the growth of keratinocytes in subsequent passages (Figure 2H). It is necessary to select a commercial medium that contains a fibroblast growth inhibitor to eliminate such contamination in the culture. Because the mouse palate and other oral mucosa have relatively small sizes, the initial cell yield from one mouse may be low. Therefore, the entire culture period of this protocol-until the cryopreservation stage-is longer than that for primary skin keratinocytes. Isolated oral keratinocytes are best used within 10 passages, as more extended culture periods could change the cell properties and lower the number of stem cells.
The current method showed that mouse oral keratinocytes exhibited a tightly packed, cobblestone morphology and formed monolayer colonies under proliferative conditions. They also showed high expression of the basal markers α6-integrin, K14, and stem cell marker p63. In future studies, in addition to immunofluorescence staining, RNA-sequencing, RT-PCR, and western blot analyses will be used to verify the cellular heterogeneity and purity of oral keratinocytes, which will further enhance our understanding of the nature of these cells.
After 2-3 passages, oral keratinocytes were stable enough to be used in further functional experiments. Importantly, this culture protocol can be combined with transgenic mouse lines, including gene knockout, Cre-loxP, and tet-inducible systems, and can also be used in cellular and molecular assays. Thus, the present protocol provides researchers with a fundamental and efficient method that could be used to understand oral keratinocyte stem cell biology further.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Grant-in-Aid for Scientific Research (B) (20H03266) (to A.S.), Grant-in-Aid for Early-Career Scientists (18K14709) (to A.S.), AMED under Grant Number JP21gm6110016 and 21bm0704067 (to A.S.), and research grants from the Takeda Science Foundation (to A.S.). We thank the Center for Animal Resources and Development at Kumamoto University and the Animal Resource Center at the University of Tsukuba for their excellent mouse care. We thank the IRCMS core facility at Kumamoto University for its support in confocal imaging.
Materials
| 0.025% Trypsin/EDTA | Gibco | R001100 | |
| 0.05% Trypsin/EDTA, phenol red | Gibco | 25300062 | |
| 0.4w/v% Trypan Blue solution | Wako | 207-17081 | |
| 10 mL Serological pipet | Falcon | 357551 | |
| 100 µm Nylon cell strainer | Falcon | 352360 | |
| 15 mL sterile conical tube | Falcon | 352096 | |
| 2 mL Aspirating pipet | Falcon | 357558 | |
| 35 mm Cell culture dish | Corning | 353801 | |
| 50 mL sterile conical tube | Falcon | 352070 | |
| 60 mm Cell culture dish | Corning | 353802 | |
| 6-well Tissue Culture plate | Falcon | 353046 | |
| 96 Well Culture plate (U bottom) | Falcon | 353077 | |
| Antibiotic-Antimycotic 100x | Gibco | 15240062 | |
| Biocoat Collagen I cellware 60mm dish | Corning | 356401 | |
| Blunt Forceps | AS ONE | 1-8187-03 | |
| Butorphanol | Meiji Seika Pharma | Vetorphale | |
| CoolCell LX | Corning | 432002 | Cryogenic storage |
| Cotton | AS ONE | 63-1452-97 | |
| Cover slips 22 x 22 μm square | Matsunami Glass Ind. | C022221 | |
| Cryogenic Vial 1.2 mL | Thermo Scientific | 5000-0012 | |
| Defined Trypsin Inhibitor (DTI) | Gibco | R007100 | |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | 276855-100ML | |
| Donkey anti-Rabbit IgG (H+L), Alexa Fluor 555 | Invitrogen | A31572 | |
| Donkey anti-Rat IgG (H+L), Alexa Fluor 488 | Invitrogen | A21208 | |
| Donkey serum | Sigma-Aldrich | D9663-10ML | |
| EpiLife Defined Growth Supplement (EDGS) | Gibco | S0120 | |
| EpiLife, with 60 µM calcium | Gibco | MEPI500CA | |
| Fetal Bovine Serum (FBS) | Gibco | 26140079 | |
| Fine forceps | BRC Bio Research Center | PRI13-3374 | |
| Goat anti-PDGF Receptor α | R&D Systems | AF1062 | |
| Goat serum | Sigma-Aldrich | G9023-10ML | |
| Half-curved forceps | BRC Bio Research Center | PRI13-3376 | |
| Hemocytometer | Hirschmann Laborgeräte | 8100204 | |
| Hoechst | Sigma-Aldrich | B2261 | |
| Iris Scissors | Muromachi Kikai | 14090-09 | |
| Medetomidine | Nippon Zenyaku Kogyo | Domitor | |
| Midazolam | Astellas Pharma | Dormicum | |
| No.15 Disposable scalpel | Feather | 219AABZX00136000 | |
| Paraformaldehyde | Wako | 162-16065 | |
| Phosphate Buffered Saline 1x, pH 7.4 | Gibco | 10010049 | |
| Povidone-iodine | Y's Square | 872612 | |
| Rabbit anti-Cytokeratin 13 antibody | Abcam | ab92551 | |
| Rabbit anti-K14 | BioLegend | 905301 | |
| Rabbit anti-p63 antibody | Abcam | ab124762 | |
| Raspatorium #14 | AS ONE | 8-4599-01 | |
| Rat α6-integrin | BD Biosciences | 553745 | |
| Triton X-100 | Wako | 581-81705 | |
| Type I Collagen coated 24-well plate | Corning | 354408 | |
| Type I Collagen coated 60mm dish | Corning | 356401 | |
| Type I Collagen coated 6-well plate | Corning | 355400 |
Referenzen
- Presland, R. B., Jurevic, R. J. Making sense of the epithelial barrier: what molecular biology and genetics tell us about the functions of oral mucosal and epidermal tissues. Journal of Dental Education. 66 (4), 564-574 (2002).
- Squier, C. A., Kremer, M. J. Biology of oral mucosa and esophagus. Journal of the National Cancer Institute Monographs. (29), 7-15 (2001).
- Jones, K. B., Klein, O. D. Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. International Journal of Oral Science. 5 (3), 121-129 (2013).
- Winning, T. A., Townsend, G. C. Oral mucosal embryology and histology. Clinics in Dermatology. 18 (5), 499-511 (2000).
- Asaka, T., Akiyama, M., Kitagawa, Y., Shimizu, H. Higher density of label-retaining cells in gingival epithelium. Journal of Dermatological Science. 55 (2), 132-134 (2009).
- Bickenbach, J. R. Identification and behavior of label-retaining cells in oral mucosa and skin. Journal of Dental Research. 60, 1611-1620 (1981).
- Bickenbach, J. R., Mackenzie, I. C. Identification and localization of label-retaining cells in hamster epithelia. Journal of Investigative Dermatology. 82 (6), 618-622 (1984).
- Byrd, K. M., et al. Heterogeneity within stratified epithelial stem cell populations maintains the oral mucosa in response to physiological stress. Cell Stem Cell. 25 (6), 814-829 (2019).
- Jones, K. B., et al. Quantitative clonal analysis and single-cell transcriptomics reveal division kinetics, hierarchy, and fate of oral epithelial progenitor cells. Cell Stem Cell. 24 (1), 183-192 (2019).
- Willberg, J., Syrjanen, S., Hormia, M. Junctional epithelium in rats is characterized by slow cell proliferation. Journal of Periodontology. 77 (5), 840-846 (2006).
- Tanaka, T., et al. Identification of stem cells that maintain and regenerate lingual keratinized epithelial cells. Nature Cell Biology. 15 (5), 511-518 (2013).
- Compton, C. C., et al. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Laboratory Investigation. 60 (5), 600-612 (1989).
- Gallico, G. G., O’Connor, N. E., Compton, C. C., Kehinde, O., Green, H. Permanent coverage of large burn wounds with autologous cultured human epithelium. New England Journal of Medicine. 311 (7), 448-451 (1984).
- Guo, Z., et al. Building a microphysiological skin model from induced pluripotent stem cells. Stem Cell Research & Therapy. 4, 2 (2013).
- O’connor, N. E., Mulliken, J. B., Banksschlegel, S., Kehinde, O., Green, H. Grafting of burns with cultured epithelium prepared from autologous epidermal-cells. Lancet. 1 (8211), 75-78 (1981).
- Hennings, H., et al. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 19 (1), 245-254 (1980).
- Caldelari, R., Suter, M. M., Baumann, D., De Bruin, A., Muller, E. Long-term culture of murine epidermal keratinocytes. Journal of Investigative Dermatology. 114 (5), 1064-1065 (2000).
- Lichti, U., Anders, J., Yuspa, S. H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nature Protocols. 3 (5), 799-810 (2008).
- Yano, S., Okochi, H. Long-term culture of adult murine epidermal keratinocytes. British Journal of Dermatology. 153 (6), 1101-1104 (2005).
- Iglesias-Bartolome, R., et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Science Translational Medicine. 10 (451), (2018).
- Papagerakis, S., et al. Oral epithelial stem cells – implications in normal development and cancer metastasis. Experimental Cell Research. 325 (2), 111-129 (2014).
- Szpaderska, A. M., Zuckerman, J. D., DiPietro, L. A. Differential injury responses in oral mucosal and cutaneous wounds. Journal of Dental Research. 82 (8), 621-626 (2003).
- Chen, L., et al. Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics. 11, 471 (2010).
- Chen, L., Gajendrareddy, P. K., DiPietro, L. A. Differential expression of HIF-1alpha in skin and mucosal wounds. Journal of Dental Research. 91 (9), 871-876 (2012).
- Simoes, A., et al. Differential microRNA profile underlies the divergent healing responses in skin and oral mucosal wounds. Scientific Reports. 9 (1), 7160 (2019).
- Turabelidze, A., et al. Intrinsic differences between oral and skin keratinocytes. PLoS One. 9 (9), 101480 (2014).
- Aasen, T., Izpisua Belmonte, J. C. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nature Protocols. 5 (2), 371-382 (2010).
- Izumi, K., Tobita, T., Feinberg, S. E. Isolation of human oral keratinocyte progenitor/stem cells. Journal of Dental Research. 86 (4), 341-346 (2007).
- Liu, Z., et al. A simplified and efficient method to isolate primary human keratinocytes from adult skin tissue. Journal of Visualized Experiments: JoVE. (138), (2018).
- Hatakeyama, S., et al. Establishment of gingival epithelial cell lines from transgenic mice harboring temperature sensitive simian virus 40 large T-antigen gene. Journal of Oral Pathology & Medicine. 30 (5), 296-304 (2001).
- Ookura, T., et al. Fibroblast and epidermal growth factors modulate proliferation and neural cell adhesion molecule expression in epithelial cells derived from the adult mouse tongue. In Vitro Cellular & Developmental Biology – Animal. 38 (6), 365-372 (2002).
- Parikh, N., Nagarajan, P., Sei-ichi, M., Sinha, S., Garrett-Sinha, L. A. Isolation and characterization of an immortalized oral keratinocyte cell line of mouse origin. Archives of Oral Biology. 53 (11), 1091-1100 (2008).
- Brennan, J. K., Mansky, J., Roberts, G., Lichtman, M. A. Improved methods for reducing calcium and magnesium concentrations in tissue culture medium: application to studies of lymphoblast proliferation in vitro. In Vitro. 11 (6), 354-360 (1975).
- Cerqueira, M. T., Frias, A. M., Reis, R. L., Marques, A. P. Interfollicular epidermal stem cells: boosting and rescuing from adult skin. Methods in Molecular Biology. 989, 1-9 (2013).

