Metabolic Support of Excised, Living Brain Tissues During Magnetic Resonance Microscopy Acquisition
Summary
The current protocol describes a method by which users can maintain viability of acute hippocampal and cortical slice preparations during the collection of magnetic resonance microscopy data.
Abstract
This protocol describes the procedures necessary to support normal metabolic functions of acute brain slice preparations during the collection of magnetic resonance (MR) microscopy data. While it is possible to perform MR collections on living, excised mammalian tissue, such experiments have traditionally been constrained by resolution limits and are thus incapable of visualizing tissue microstructure. Conversely, MR protocols that did achieve microscopic image resolution required the use of fixed samples to accommodate the need for static, unchanging conditions over lengthy scan times. The current protocol describes the first available MR technique that enables imaging of living, mammalian tissue samples at microscopic resolutions. Such data is of great importance to the understanding of how pathology-based contrast changes occurring at the microscopic level influence the content of macroscopic MR scans such as those used in the clinic. Once such an understanding is realized, diagnostic methods with greater sensitivity and accuracy can be developed, which will translate directly to earlier disease treatment, more accurate therapy monitoring and improved patient outcomes.
While the described methodology focuses on brain slice preparations, the protocol is adaptable to any excised tissue slice given that changes are made to the gas and perfusate preparations to accommodate the tissue's specific metabolic needs. Successful execution of the protocol should result in living, acute slice preparations that exhibit MR diffusion signal stability for periods up to 15.5 h. The primary advantages of the current system over other MR compatible perfusion apparatuses are its compatibility with the MR microscopy hardware required to attain higher resolution images and ability to provide constant, uninterrupted flow with carefully regulated perfusate conditions. Reduced sample throughput is a consideration with this design as only one tissue slice may be imaged at a time.
Introduction
As magnetic resonance imaging (MRI) systems have steadily progressed to ever-higher field strengths, more details about the composition and status of living tissues have become discernable. Despite such hardware advances, MR imaging at resolutions sufficient to visualize the cellular structures of tissues is still not available in the clinic. As a result, cellular-level characteristics of tissues must be inferred when considering the content of clinical scans. Such inference requires knowledge of equivalent processes gleaned from data taken in model systems which can be observed directly. Traditionally, these models included cells from aquatic organisms such as the Xenopus laevis oocyte and Aplysia californica L7 neuron1,2. These were among the first animal cells available for observation with MR methods due to their atypically large size: approximately 1000 μm and 300 μm diameter, respectively. More recently, advances in hardware design have allowed for one of the largest examples of mammalian cells—the α-motor neuron—to be imaged using MR microscopy techniques on fixed tissue3,4. While these studies demonstrated direct visualization of mammalian cellular material using MR, the fixed samples employed differ significantly in their MR properties from live tissue and thus cannot serve as an equivalent representative model5,6. More importantly, observing MR contrast changes that occur in concert with complex biological processes requires living samples that can be perturbed and measured over the course of the imaging experiment.
To facilitate MR microscopy studies on living tissues, a protocol is presented which includes commercial microimaging hardware7 interfaced to a purpose-built, MR compatible, in-bore oxygenator and perfusion device previously described8. Unique advantages of this design include cellular-level resolution capabilities in mammalian tissues and precision control over dissolved gas content and pH at the site of tissue perfusion. Also, unlike the majority of explant MR studies which interrupt perfusion during image acquisition to avoid flow artefacts, this design supports the use of continuous perfusion during data collection which has been shown to improve the physiological condition of isolated tissues9,10. Lastly, its closed recording chamber and slice-retention hardware aid in reducing the likelihood of motion artifacts which could otherwise occur during protracted image collection.
While the current protocol describes procedures appropriate for use with acute hippocampal and cortical slices, precise control over perfusate metabolites enables this system to accommodate a wide array of diverse tissue types and experimental conditions. Limitations of this design include a reduction in sample throughput as compared to a multi-slice perfusion chamber11; however, this limitation may be overcome in the future using multi-coil arrays.
Also, while the described system can be employed in both horizontal or vertical configurations, the current protocol features its use in a vertically oriented, 600 MHz spectrometer. Any system capable of MR microimaging studies—typically narrow-bore (≤6 cm), high field (≥500 MHz) spectrometers—will accommodate the oxygenator and perfusion equipment described. However, changes to the imaging coil, gradient, probe system, or other essential imaging hardware employed may necessitate alterations to the perfusion equipment and MR scan parameters.
Protocol
All animal experiments described follow the guidelines set forth in the National Academies of Sciences' Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of Florida's Institutional Animal Care and Use Committee (IACUC). Follow all applicable rules and regulations when engaging in animal subject research.
1. Preparation of Perfusate for the Maintenance of Central Nervous System Tissues
- Make fresh artificial cerebrospinal fluid (aCSF).
- To generate 2 L of bicarbonate-buffered aCSF perfusate, measure out 1,500 mL of water purified by double distillation or reverse osmosis into a 4 L flask. Place a magnetic stir bar in the flask and agitate the liquid using a stir plate.
- Into the purified water, dissolve the following quantities of salts: 14.03 g (120 mM) sodium chloride, 4.37 g (26 mM) sodium bicarbonate, 0.41 g (1.5 mM) monobasic potassium phosphate, 0.69 g (1.4 mM) magnesium sulfate heptahydrate, 0.59 g (2 mM) calcium chloride dihydrate, 0.45 g (3 mM) potassium chloride, and 3.6 g (10 mM) glucose.
NOTE: The chemical contents of the perfusate will differ depending on the specific metabolic requirements of the tissue and the desired conditions of the experiment. - Mix this solution thoroughly until all salts have dissolved. Adjust volume to 2 L using additional purified water.
- Test the osmolality of the aCSF using a freezing point depression osmometer. Adjust to 300 mOsm/kg using sorbitol. Addition of 324 mg sorbitol to 2 L of 299 mOsm/kg aCSF perfusate will increase osmolality to 300 mOsm/kg (approximately 1 mOsm per 324mg).
NOTE: The aCSF can be stored at 4 °C in an air-tight, sealed bottle for a period not to exceed 24 h.
2. Set Up the Perfusion System
- Prepare the aCSF perfusate.
- Equilibrate aCSF to ambient temperature (~23 °C) while gassing with 95% carbogen (95% O2, 5% CO2; 1/16 L/min) via direct bubbling for no less than 1 h.
NOTE: Different tissue types or desired experimental conditions may require adjustment of the perfusate temperature or gas contents. The desired conditions for dissolved gas content in the perfusate can be controlled precisely by varying the percent concentrations of individual components of the supply gas (Figure 1). - Once the aCSF reaches the required temperature and carbogen saturation, confirm that the pH is in the physiological range (7.3 – 7.4). Continue to bubble gas directly into the perfusate reservoir (1/16 L/min) throughout the course of experiment in order to maintain proper dissolved oxygen content and pH conditions conducive to healthy tissue metabolism.
- Equilibrate aCSF to ambient temperature (~23 °C) while gassing with 95% carbogen (95% O2, 5% CO2; 1/16 L/min) via direct bubbling for no less than 1 h.
- Prime the perfusion lines.
- Submerge the inlet tube connected to the peristaltic micro-pump into the prepared aCSF (300 mOsm, pH 7.3 – 7.4, continuously gassed).
- Pass the oxygenator device (Figure 2) and perfusion lines through both the magnet bore and gradient coil stack (top to bottom) and set the coils aside until probe assembly. Hang the oxygenator above a beaker to catch any aCSF effluent.
NOTE: Depending on the MRI system design, perfusion lines may need to be passed through the magnet bore and gradient coils or probe body base prior to priming the system with aCSF. Confirm that perfusion line placement will not interfere with the assembly of the probe body or insertion of the probe into the bore before beginning the priming procedure. - Confirm the intended flow rate (2 mL/min) is selected and begin filling the perfusion lines by switching on the pump.
- Invert the empty, in-line bubble trap so that aCSF will displace the air contained inside.
- Connect the oxygenator's gas port to a second source of carbogen (either a manifold or a secondary carbogen cylinder) and set a flow rate of 1/16L/min.
- Confirm that carbogen is flowing over the oxygenator's gas-exchange membrane by dipping the exhaust port on top of the oxygenator into water.
- Once aCSF is detected dripping from the perfusion chamber, purge any visible gas bubbles from the inflow lines by manual agitation.
- Confirm the oxygenator is operating correctly by submerging the oxygen electrode in the aCSF flowing from the perfusion chamber.
NOTE: The reading on the oxygen meter should approximate the percentage of oxygen contained within the supplied gas.
3. Tissue Preparation
- Euthanasia
- Place a rat (125 – 150 g) into the induction chamber of an anesthesia machine and expose to 4% isoflurane in an oxygen carrier gas at a rate of 1 L/min for 4 min.
- Remove the unconscious rat from the anesthesia chamber.
- Perform a righting reflex test by placing the rat in the supine position. Check for any movements—head turning, leg lifting, spine flexing, etc.—indicative that the animal is turning onto its stomach.
- Perform an eye-blink reflexive test by touching the open eye of the animal using a cotton swab. Check for any response—lid closure, muscle twitching, etc.—to this sensory input.
- Lastly, perform a limb-withdraw reflex test by pinching the skin between the toes of the rat's outstretched back leg using tweezers or hemostats. Check for flexion of the leg.
NOTE: In the case of a positive reflexive test result, do not proceed with euthanasia. - In the event that any of the three reflex tests elicits a response, return the rat immediately to the anesthesia chamber and allow for an additional 2 min of exposure to 4% isoflurane.
- Perform all three reflexive tests over in their entirety. Repeat the 2 min anesthesia exposure as necessary and only proceed once a complete lack of response to all three reflexive tests has been observed.
- Euthanize the rat via guillotine decapitation.
- Brain resection
- Remove rat brain by gross dissection. Start with the head in the prone position. Using scissors, cut rostrally through the skin from the back of the neck to the nose and expose the skull.
- Remove soft tissues from the surface of the skull with a blunt probe.
- Working from the caudal end, remove the occipital, parietal, and frontal bones of the cranium using rongeurs.
- Retract the dura by cutting along the longitudinal fissure with micro scissors and peeling back the layer from both hemispheres.
- Remove the brain from the skull by turning the head to the supine position and severing the cranial nerves on the ventral side.
- Slice isolation
- Return the brain to the prone position and isolate the central portion containing the hippocampus by removing all tissues caudal to the transverse fissure and rostral to the fimbria. Make two cuts along the transverse plane (i.e. coronal slices) with a straight-edged razor.
- Affix the brain's caudal-most plane to the center of a vibratome cutting bath using cyanoacrylate glue.
- Add ice-cold, carbogen bubbled aCSF to the vibratome cutting bath and place the nylon retention hardware inside.
- Cut 300 μm thick slices to obtain 3 to 4 usable slice preparations per hemisphere (6 to 8 total).
- Isolate a hippocampus or cortical section from one hemisphere and trim the slice so that it fits within the microcoil's 5 mm diameter tissue well.
4. Sample Positioning and Perfusion System Assembly
- Coil preparation
- Fill the microcoil's tissue chamber with oxygenated aCSF from the vibratome's cutting bath using a transfer pipette.
- Take the trimmed brain sample and position the region of interest (eg. pyramidal cell layer) over the microcoil using a dissecting scope.
- Insert the tissue retention device (nylon mesh net affixed to nylon washer) to retain sample position throughout the imaging experiment.
NOTE: Proceed quickly through the sample positioning procedure to minimize exposure to intense light. Provide additional oxygenated aCSF as needed using a transfer pipette.
- Assembling the in-bore oxygenator, microcoil and probe
- Secure the modified microcoil assembly (Figure 3) in a table clamp and affix the in-bore oxygenator device by inserting the acetal support peg into the hole on top of the coil.
- Seal the perfusion system by placing the perfusion chamber over the microcoil's tissue well and cinching the two together using a miniature cable tie.
- Trim the excess length from the cable tie using wire cutters.
NOTE: Upon successful sealing, perfusate should be observed exiting through the outflow lines and no leakage should be evident around the silicone seal of the perfusion chamber. Failure to confirm these conditions prior to probe assembly could result in serious damage to imaging hardware. - Once aCSF can be seen dripping into the waste reservoir, take the microcoil and oxygenator and attach the assembly to the top of the imaging probe body.
- Slide the gradient stack over the assembly and seat the gradient on top of the probe. Photographs detailing the relative placement and proper connection of coil, oxygenator and probe hardware components are provided (Figure 4).
5. Performing the MR Image Collection
- Inserting the assembled probe into the magnet bore
- Place the probe body in close proximity to the spectrometer bore opening at the bottom of the magnet.
- Retract the excess length of perfusion lines through the bore opening at the top of the magnet.
- Once all of the available slack has been taken up from the perfusion lines, advance the probe body into the magnet bore at the base while simultaneously removing more slack from the perfusion lines from the top of the bore.
- Thread the two securing screws at the base of the probe into the corresponding slots of the shim stack.
- Before proceeding, check that perfusion lines were not pinched or kinked during probe insertion by confirming aCSF outflow into the waste reservoir.
NOTE: Failure to confirm perfusate outflow can result in permanent damage to perfusion lines and risks catastrophic damage to MR imaging hardware. - In the event no perfusate is seen exiting the return line into the waste reservoir, remove the probe body and confirm that perfusate flow has recommenced prior to attempting reinsertion. A schematic layout of the assembled perfusion system and imaging spectrometer has been described previously8.
- Connecting the Probe Body
- Attach the inflow and outflow water lines from the gradient chiller.
- Turn on the pump for gradient chiller and confirm the water temperature setting (19 °C).
- Attach the air hose from the air chiller unit to the probe body using a hose clamp.
- Turn the flow knob on the air chiller unit to the "1" position.
- Attach the thermocouple wire to the probe body.
- Attach the coaxial cable from the preamp to the radio frequency (RF) input/output on the probe body's proton (H) channel.
- Attach the power cable from the gradient amplifiers to the probe body.
- Inside the RF cabinet, turn on the power to the B0 compensation unit, all three gradient amplifiers (x,y,z), and the master unit.
- Preparing the Spectrometer
- Set the intended bore temperature using the variable temperature adjustment module at the console.
- Match (impedance) and tune (frequency) the RF circuit by adjusting the variable capacitors within the probe. This is accomplished by manipulating the wands at the base of the probe body.
- Adjust the current settings for the spectrometer's shim coils to maximize the magnetic field homogeneity at the sample.
- Begin Image Collection
- Collect pilot images to determine the spatial position of your sample within the magnet bore. Typical parameters for a two dimensional gradient echo are as follows (TR/TE = 100/4 ms, averages = 1, pulse angle = 30o, time = 6 sec, matrix = 64 x 64, field of view = 0.3 x 0.3 cm, resolution = 47 x 47 μm).
- Collect diffusion-weighted pilots in order to confirm proper scan geometry and tissue position if applicable. Typical parameters for a two dimensional diffusion-weighted pilot scan are as follows (TR/TE = 2000/11.6 ms, time = 4.3 min, Δ = 6 ms, δ = 1 ms, averages = 1, b = 1200 (1860 effective) s/mm2, matrix = 64 x 64, field of view = 0.2 x 0.2 cm, resolution = 31 μm).
- Collect a diffusion-weighted time series to determine the stability characteristics of the acute slice preparation. Typical parameters for a two dimensional diffusion weighted image are as follows (TR/TE = 2000/11.6 ms, time = 1.5 h, Δ = 6 ms, δ = 1 ms, averages = 42, b = 1200 s/mm2, matrix = 64 x 64, field of view = 0.2 x 0.2 cm, resolution = 31 μm). Note: Characterizing stability in a given system will vary from the described protocol depending on factors such as the MR contrast employed (e.g. T1,T2, diffusion, susceptibility), the physical perturbation studied, and the MR signal change per unit time resulting from said perturbation.
Representative Results
Perfusate Preparation
Upon successful employment of the in-bore oxygenation device, gases present in the supplied carbogen will reach 100% saturation conditions within the aCSF perfusate. This can be demonstrated by varying the oxygen concentration of the supplied gas and measuring the change in dissolved oxygen content in the aCSF perfusate within the perfusion chamber using an oxygen meter (Figure 1)8. According to Henry's law, the amount of dissolved gas that is in equilibrium with a liquid sample is directly proportional to the partial pressure of that gas provided that the temperature remains constant12. Using this knowledge and precision gas standards, it is possible to quantify the amount of dissolved oxygen contained within an aCSF sample as described. This is achieved by calibrating the oxygen meter using saturated solutions (directly bubbled for 1 h or more) of aCSF being exposed to gasses of known composition: one gas with high oxygen concentration such as carbogen (95% O2) and another with low oxygen concentration such as nitrogen (0% O2). Afterward, measurements can be taken by submerging the tip of the oxygen electrode into a sample. Confirmation that the in-bore oxygenator is functioning properly can be achieved by measuring the effluent from the perfusion well. The percent dissolved oxygen as measured by the oxygen meter should match the percent concentration of oxygen delivered in the supply gas. If the measured values are lower than those in the supply gas, this would suggest a hardware failure that could lead to metabolic insufficiency in the tissue slice.
Sample Appearance and Behavior
Acute slice preparations that receive perfusion sufficient enough to supply necessary metabolites and carry away metabolic wastes soon reach a state of relative stability. From that point, acute slices can be subjected to external perturbation and their responses to these changes can be measured for scientific study. For MR experiments, tracking the signal of interest over time is a commonly used practice to demonstrate the relative stability of acute slice preparations13. The diffusion-weighted signal is especially sensitive to changes in a tissue's water mobility, content, and distribution, as can be appreciated by the use of this contrast mechanism to detect infarcts in ischemic stroke14,15. Plotting the normalized diffusion signal over time in acute cortical slices maintained under a variety of perfusion conditions demonstrates relatively stability (2 ± 3% over 15.5 h) after tissue isolation is achieved (Figure 5). Diffusion signal stability was maintained regardless of perfusion conditions (intermittent or continuous) or MRI scan length (short [4 min] or long [1.5 h])8. If slices do not exhibit signal stability over time, such as the sharp diffusion signal increase observed in living cortex that did not receive perfusion, this is suggestive of suboptimal experimental conditions. Perturbation experiments should not be attempted prior to confirmation of stable signal conditions in slice preparations.
In addition to signal stability, correct sample positioning must be confirmed at the time of image collection. Even though sample position is controlled during tissue placement at the dissecting microscope, shifts in sample position may occur during the assembly of the perfusion apparatus, or due to rough handling of the coil or probe prior to insertion into the magnet. Confirmation of proper hippocampal placement can be achieved by collecting short (2 min), pilot scans with diffusion contrast (Figure 6). Because the pyramidal cell layer is more sensitive to diffusion weighting than the adjacent hippocampal laminae, this structure will appear as a darker band in diffusion-weighted images. Setups that do not display this characteristic feature contain off-center samples and will likely need to be repeated.
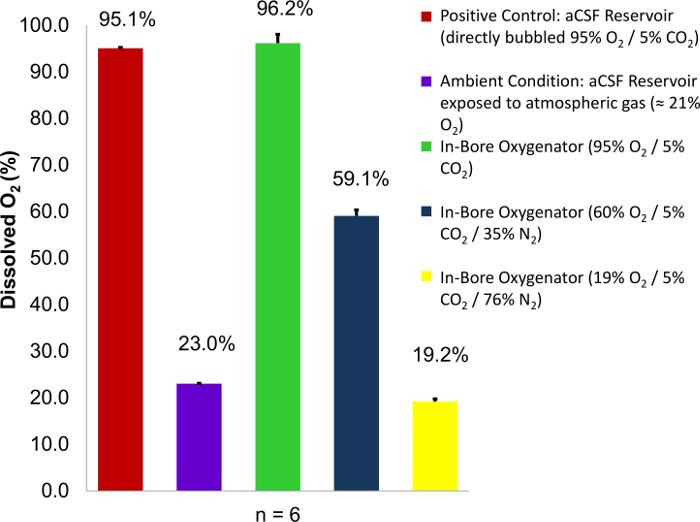
Figure 1: Dissolved oxygen content of aCSF perfusate as a function of percent O2 content in supplied gas. Carbogen mixtures containing variable concentrations of oxygen (95%, 60%, and 19%) are employed as a supply gas. Percent dissolved oxygen readings are then taken from the perfusion well and compared to two known controls: a perfusate reservoir directly bubbled with carbogen (95% O2), and a perfusate reservoir exposed to atmospheric conditions (23% O2). In each case, the percent oxygen saturation at the site of tissue perfusion approaches 100% of the O2 concentration within the carbogen mixture used. Error bars are equal to the standard deviation of the sample means. Figure reproduced with permission from the original article8. Please click here to view a larger version of this figure.
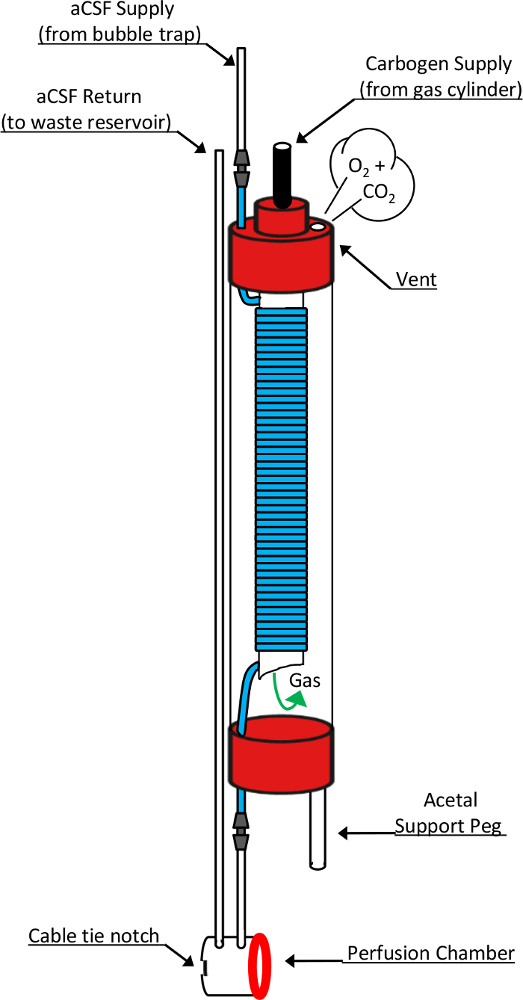
Figure 2: Schematic drawing of the in-bore oxygenator and perfusion chamber. This diagram shows detailed design elements responsible for the function of these critical devices. Fresh perfusate that has been pumped through a bubble trap enters the oxygenator through the top of a 10 mm NMR tube. In doing so, it transitions into a highly gas permeable silicone tubing (blue segment) that is coiled around an open-ended, 5 mm NMR tube nested inside. Carbogen gas supplied through the top of the 5mm tube enters the chamber through the open-ended bottom and passes over the coiled silicone tubing before exiting the oxygenator through a vent hole in the 10 mm tube cap. During this exposure, the perfusate flowing through the silicone tube becomes saturated with the chemical components of the supplied gas mixture. Upon exiting the oxygenator, perfusate passes directly into the perfusion chamber before entering the return line leading to a waste collection reservoir. Other components critical to this design include the acetal support peg which allows the oxygenator to stand vertically atop the modified RF microcoil, a silicone washer (red ring) which forms the liquid-tight seal between the oxygenator's perfusion chamber and the microcoil's tissue well, and the cable tie notch which accommodates placement of a cable tie used to form this reversible seal. This figure has been modified and reproduced with permission from the original article8. Please click here to view a larger version of this figure.
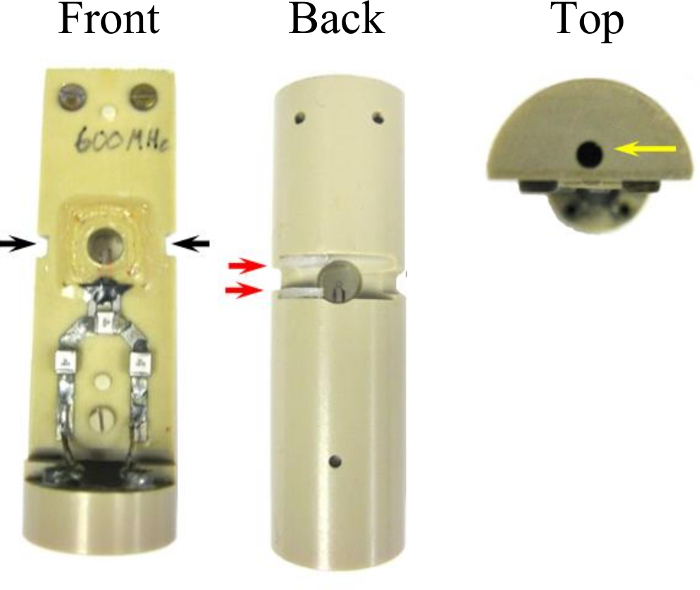
Figure 3: Modifications to the microcoil assembly which permit interface to the in-bore oxygenator. Two grooves 3.0 mm x 1.5 mm (black arrows) were cut into the side of the assembly that accommodate the width of a cable tie used to seal the perfusion chamber. A channel (15 mm x 3 mm x 4 mm) connects the groves across the back face of the coil. Two nylon spaces placed in the sides of the channel (red arrows) act as a catch for the cable tie head which eases the sealing procedure. A hole (2 mm x 14 mm) drilled in the top of the coil assembly (yellow arrow) joins to the acetal support peg to secure the oxygenator. This figure has been modified and reproduced with permission from the original article8. Please click here to view a larger version of this figure.
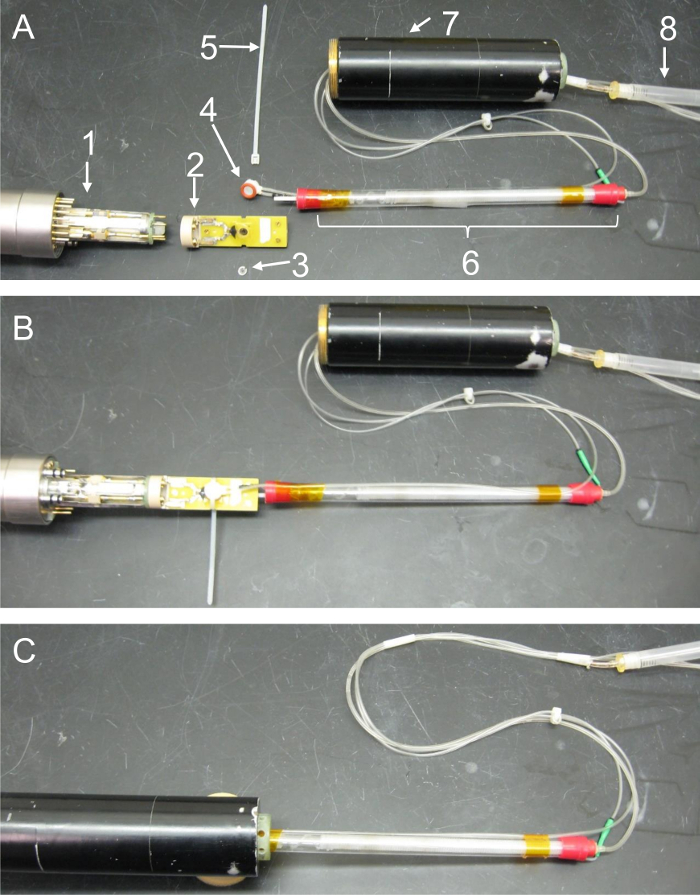
Figure 4: Photo montage detailing the relative placement and proper assembly of microcoil, oxygenator, and probe components. These images feature key hardware components of the in-bore oxygenator and microperfusion device and illustrate how the separate parts interface with one another. (A) Exploded-view photo showing the relative placement of all components prior to sealing of the tissue well or assembly of the probe body. Care has been taken to display the relative location of parts accurately; however, a section of the perfusion lines has been doubled back in this series so that all components fit within the image frame. (1 = probe head, 2 = microcoil assembly, 3 = nylon tissue retention ring, 4 = perfusion well, 5 = cable tie, 6 = in-bore oxygenator, 7 = gradient coils, 8 = bubble trap). (B) Components following coil and oxygenator assembly. In this image, the nylon retention ring has been placed within the microcoil's tissue well to secure a sample. The acetal support peg on the base of the oxygenator has been secured in the corresponding hole on top of the microcoil. The silicone gasket on the open end of the perfusion well has been placed over the tissue well, and a cable tie has been tightened around these components to seal the perfusion chamber. Lastly, the base of the microcoil has been connected to the top of the probe head. (C) Components following probe assembly. In the last panel, excess length from the cable tie has been trimmed flush with the microcoil. The gradient coil stack is then slid into position by carefully advancing the cylinder towards the probe while passing the excess perfusion lines, oxygenator, and microcoil through its hollow center. Once the gradients are connected to the probe head, they're held in place by screwing the securing collar on the probe over the threaded base of the gradients. Please click here to view a larger version of this figure.
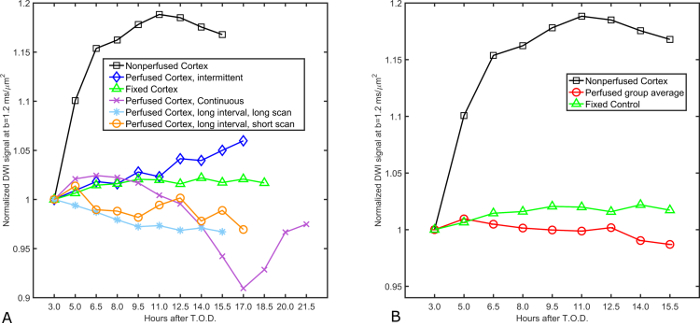
Figure 5: Diffusion signal stability in superfused acute cortical slices. (A) Normalized diffusion signal values in four acute slices subjected to differing superfusion paradigms are plotted over time for a period of up to 21.5 h following euthanasia. Slices remain within ± 5% of their initial diffusion signal measurement for a period of 15.5 h following euthanasia regardless of whether superfusion is continuous or intermittent and independent of the MR scan length (1.5 h or 4 min). Signal recordings taken from formaldehyde-fixed cortex serve as the positive control (n = 1) for stability due to the static, unchanging nature of fixed tissue samples. Conversely, diffusion signal measured in a live slice absent of superfusion support (n = 1) serves as a control for metabolic deficiency. Experiment parameters of the different superfusion trials are as follows: continuous (superfusion always on, time per scan = 1.5 h), intermittent (superfusion on for 10 min interval between scans, time per scan = 1.5 h), Long interval, long scan (superfusion on during scan, but paused for 10 min between scans, time per scan = 1.5 h), Long interval, short scan (superfusion on for 1.5 h interval between scans, time per scan = 4 min). (B) Analyzed data showing group means of the four live-slice superfusion experiments from panel (A). The diffusion signal profile from the grouped, superfused cortical slices exhibits little variation over time (2 ± 3% over 15.5 h) whereas the non-perfused control (n = 1) exhibits dramatic signal instability early in the experiment (15% by 6.5 h). This figure has been modified and reproduced with permission from the original article8. Please click here to view a larger version of this figure.
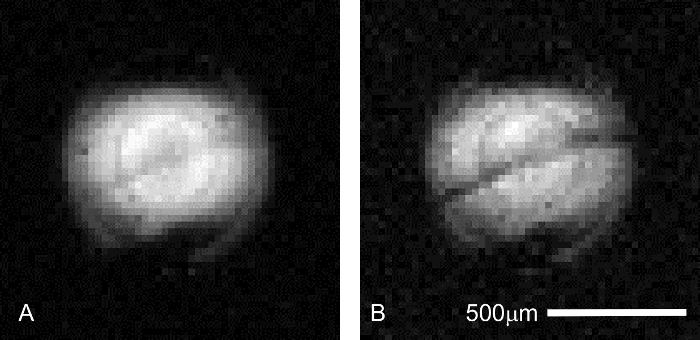
Figure 6: Confirmation of hippocampal slice placement during pilot imaging. Prior to running an extended MR microscopy session, correct placement of the sample is critical to ensure resources such as scanner time and expensive perfusate additives are not wasted. The pyramidal cell layer in the CA1 region of the hippocampus can be visualized in faster (4.3 min), lower resolution (31 μm x 31 μm in-plane) pilot scans to ensure the tissue of interest is placed correctly in relation to the micro-coil. Scan parameters common to both images are as follows: TR/TE = 2000/11.6 ms, Δ = 6 ms, δ = 1 ms, averages = 1. (A) b = 0 (227 effective) s/mm2. In this preliminary scan, the stratum pyramidale is only just visible as a grey, diagonal band centered in the coil's excitation profile. (B) b = 1,200 (1,860 effective) s/mm2. At higher diffusion weighting, interlamellar contrast increases as the pyramidal cell layer becomes darker than tissues in the adjacent laminae (above: stratum oriens; below: stratum radiatum). Please click here to view a larger version of this figure.
Discussion
The current protocol describes procedures necessary for standard metabolic maintenance of acute brain slice preparations undergoing magnetic resonance microscopy. This procedure is the only method currently available that allows visualization of living mammalian tissues with MR at resolutions capable of resolving cells. While the perfusate conditions described are tailored specifically to central nervous system tissues, the protocol is widely adaptable to any manner of living tissue preparation through adjustments of perfusate and gas constituents as well as perfusion flow rate and temperature.
The most common problems likely to be encountered during the described procedures include those related to failures in metabolite supply. Precipitation of calcium salts can occur inside the aCSF during gaseous insufficiency as a result of failures in the bicarbonate buffering system. Such precipitates can clog the perfusion lines and result in severe hardware damage. If salt precipitates are observed in the perfusate following probe assembly, cease perfusion flow immediately by turning off the peristaltic pump. Confirm presence of sufficient sodium bicarbonate levels (4.37 g / 2 L) in perfusate, CO2 levels (5.0%) in supply gas, and carbogen gas flow (1/16 L/min) into both reservoir and oxygenator. Finally, confirm pH levels are stabilized in the physiological range (7.3 – 7.4). In the event that oxygen gas and pH levels are still not regulated appropriately, the gas-exchange membrane should be replaced.
If slices do not exhibit signal stability over the intended experimental time-course, confirm that the correct chemical constituents are present in the aCSF mixture and that the correct osmolality (300 mOsm) and pH (7.3 – 7.4) are maintained. Also, ensure carbogen gas is being supplied to the perfusate reservoir and oxygenator at 1/16 L/min. If these steps do not correct perfusate conditions, replacement of the gas-exchange membrane is advised. If tissue stability is not achieved after troubleshooting the perfusate conditions, consider refinement of the surgical protocol with a focus on minimizing the time interval between tissue harvest and perfusion application.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the National Institutes of Health (1R21NS094061-01A1) (NIH 1R01EB012874-01) (S10RR031637), and the National Science Foundation (cooperative agreement No. DMR-1157490) through the National High Magnetic Field Laboratory (NHMFL) Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility at UF and the State of Florida.
Materials
| Perfusate Preparation | |||
| Osmette A | Precision Systems Inc. | 5002 | freezing point depression osmometer |
| Stir Plate Type 1000 | Barnstead/Thermodyne | SPA1025B | magnetic stir plate with heating element |
| Accumet Basic pH Meter | Fisher Scientific | AB15 | pH Meter |
| pH Probe | Fisher Scientific | 13-620-AP61 | probe for pH measurement |
| Oxygen Meter | Microelectrodes Inc. | OM-4 | meter for sampling the oxygen content of gasses or the disolved oxygen content of liquid perfusates |
| Oxygen Electrode | Microelectrodes Inc. | MI-730 | microprobe for the oxygen meter |
| Scale | Denver Instrument Co. | A-160 | microscale for weighing chemical components |
| Name | Company | Catalog Number | Comments |
| Slice Preparation | |||
| Lancer Vibratome | Ted Pella Inc. | Series 1000 | vibratory tissue slicer |
| Disecting Microscope | Carl Zeiss Inc. | OPMI 1-FC | tabletop, binocular disecting microscope |
| Name | Company | Catalog Number | Comments |
| Perfusion System | |||
| Masterflex L/S | Cole-Parmer | 7523-50 | peristaltic micro perfusion pump |
| Oxygen Regulators x 2 | Victor Medical | VMG-05LY | device for regulating gas flow |
| e-sized carbogen cylinders x 2 | Airgas | gas tanks containing carbogen gas | |
| in-bore oxygenator | developed in house | device responsible for pH and oxygen regulation in the perfusate | |
| Name | Company | Catalog Number | Comments |
| MR Imaging Hardware | |||
| Micro Surface Coil (200mm dia., modified) | Bruker Biospin | B6371/0001 | four-turn micro (200mm dia) surface-style radiofrequency coil |
| Micro 5 probe body | Bruker Biospin | Z3395 | microimaging probe used in the 600 MHz spectrometer |
| Micro 5 gradient coils | Bruker Biospin | M81111 | gradient coil stack used with micro 5 probe body |
| 600 MHz Spectrometer | Oxford Instruments | superconducting magnet (14.1T) used for MR image generation | |
| Imaging Console | Bruker Biospin | Avance III | support and control hardware including gradient amplifiers, preamps, & workstation used for MR image generation |
| Air Blower | Bruker Biospin | BCU-II, -80/60 | Air chiller unit used in conjunction with the probe's heating coil to regulate temperature inside the magnet bore |
| Gradient Chiller | Thermo Scientific | Neslab Merlin M33 | Water chiller used to disipate heat generated by the gradient coils |
Referenzen
- Aguayo, J. B., Blackband, S. J., Schoeniger, J., Mattingly, M. A., Hintermann, M. Nuclear magnetic resonance imaging of a single cell. Nature. 322, 190-191 (1986).
- Schoeniger, J. S., Aiken, N., Hsu, E., Blackband, S. J. Relaxation-time and diffusion NMR microscopy of single neurons. J. Magn. Reson. B. 103, 261-273 (1994).
- Flint, J. J., et al. Magnetic resonance microscopy of mammalian neurons. Neuroimage. 46, 1037-1040 (2009).
- Flint, J. J., et al. Magnetic resonance microscopy of human and porcine neurons and cellular processes. Neuroimage. 60, 1404-1411 (2012).
- Kamman, R. L., Go, K. G., Stomp, G. P., Hulstaert, C. E., Berendsen, H. J. C. Changes of Relaxation times T1 and T2 in rat tissues after biopsy and fixation. Magn. Reson. Imag. 3, 245-250 (1985).
- Shepherd, T. M., Thelwall, P. E., Stanisz, G. J., Blackband, S. J. Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magn. Reson. Med. 62, 26-34 (2009).
- Massin, C., Boero, G., Vincent, F., Abenhaim, J., Besse, P. -. A., Popovic, R. S. High-Q factor RF planar microcoils for micro-scale NMR spectroscopy. Sensor. Actuat. A-Phys. 97, 280-288 (2002).
- Flint, J., Menon, K., Hansen, B., Forder, J., Blackband, S. J. A microperfusion and in-bore oxygenator system designed for magnetic resonance microscopy studies on living tissue explants. Sci. Rep. 5, 18095 (2015).
- Khong, Y. M., et al. Novel intra-tissue perfusion system for culturing thick liver tissue. Tissue Eng. 13 (9), 2345-2356 (2007).
- Schumacher, K., Khong, Y. -. M., Chang, S., Ni, J., Sun, W., Yu, H. Perfusion culture improves the maintenance of cultured liver tissue slices. Tissue Eng. 13 (1), 197-205 (2007).
- Shepherd, T. M., Blackband, S. J., Wirth, E. D. Simultaneous diffusion MRI measurements from multiple perfused rat hippocampal slices. Magn. Reson. Med. 48, 565-569 (2002).
- Henry, W. Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures. Phil. Trans. R. Soc. Lond. 93, (1803).
- Bui, J. D., Buckley, D. L., Phillips, M. I., Blackband, S. J., Blümler, P., Blümich, B., Botto, R., Fukushima, E. Studies of perfused brain slices with MR microscopy. Spatially Resolved Magnetic Resonance. , 337-343 (1998).
- Moseley, M. E., et al. Early detection of regional cerebral ischemia in cats: Comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn. Reson. Med. 14 (2), 330-346 (1990).
- Moseley, M. E., et al. Diffusion-weighted MR imaging of acute stroke: Correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. Am. J. Neuroradiol. 11, 423-429 (1990).

