Multifunctional, Micropipette-based Method for Incorporation And Stimulation of Bacterial Mechanosensitive Ion Channels in Droplet Interface Bilayers
Summary
Bacterial mechanosensitive channels can be used as mechanoelectrical transducers in biomolecular devices. Droplet interface bilayers (DIBs), cell-inspired building blocks to such devices, represent new platforms to incorporate and stimulate mechanosensitive channels. Here, we demonstrate a new micropipette-based method of forming DIBs, allowing the study of mechanosensitive channels under mechanical stimulation.
Abstract
MscL, a large conductance mechanosensitive channel (MSC), is a ubiquitous osmolyte release valve that helps bacteria survive abrupt hypo-osmotic shocks. It has been discovered and rigorously studied using the patch-clamp technique for almost three decades. Its basic role of translating tension applied to the cell membrane into permeability response makes it a strong candidate to function as a mechanoelectrical transducer in artificial membrane-based biomolecular devices. Serving as building blocks to such devices, droplet interface bilayers (DIBs) can be used as a new platform for the incorporation and stimulation of MscL channels. Here, we describe a micropipette-based method to form DIBs and measure the activity of the incorporated MscL channels. This method consists of lipid-encased aqueous droplets anchored to the tips of two opposing (coaxially positioned) borosilicate glass micropipettes. When droplets are brought into contact, a lipid bilayer interface is formed. This technique offers control over the chemical composition and the size of each droplet, as well as the dimensions of the bilayer interface. Having one of the micropipettes attached to a harmonic piezoelectric actuator provides the ability to deliver a desired oscillatory stimulus. Through analysis of the shapes of the droplets during deformation, the tension created at the interface can be estimated. Using this technique, the first activity of MscL channels in a DIB system is reported. Besides MS channels, activities of other types of channels can be studied using this method, proving the multi-functionality of this platform. The method presented here enables the measurement of fundamental membrane properties, provides a greater control over the formation of symmetric and asymmetric membranes, and is an alternative way to stimulate and study mechanosensitive channels.
Introduction
In the past decade, the assembly of artificial lipid bilayers has been substantially advanced through the development of the droplet interface bilayer method. Known as stable and robust, DIBs imposed themselves as alternative model systems to the classical painted (Mueller) and folded (Montal-Mueller) planar bilayers1. Although the idea of using droplets to create lipid bilayers dates back to the 1960s2, it has not gained popularity until recently. The first successful attempt was reported by the Takeushi group3, followed by several studies demonstrating bilayer formation using a network of droplets by the Bayley group4-6. More recently, encapsulation techniques were proposed by the Leo group7-9, who pioneered the concept of using DIBs as building blocks of novel stimuli-responsive material systems10. In previous studies, DIBs have proved their ability to respond to electrical9,11, chemical10,12, and optical stimuli13. Various biomolecules with different stimuli-responsive functionalities have been effectively stimulated when reconstituted in the DIB10,14. In light of these successful attempts an important question is raised: could the DIB respond to mechanical stimulus when appropriate biomolecules are incorporated? The interfacial forces acting on a DIB differ from those in other bilayer system15,16. Therefore, the tension in the bilayer held by the droplets could be controlled by regulating tension at the water-lipid-oil interfaces; a concept not applicable with the painted or folded bilayer systems.
MscL channels, widely known as osmolyte release valves and fundamental elements of the bacterial cytoplasmic membrane, react to increased membrane tension17,18. In the event of hypo-osmotic shocks, several channels residing in the membrane of a small cell19 can generate a massive permeability response to quickly release ions and small molecules, saving bacteria from lysis20. Biophysically, MscL is well studied and characterized primarily through the prominent patch clamp technique21-23. Reliable structural models explaining MscL's gating mechanism24,25 are proposed based on its homolog's crystal structure26,27, modeling28, and results of extensive experimentation 24,29-31. Under an applied tension of ~10 mN/m, the closed channel which consists of a tight bundle of transmembrane helices, transforms into a ring of greatly tilted helices forming a ~28 Å water-filled conductive pore21,24,32. It has also been established that the hydrophobicity of the tight gate, positioned at the intersection of the inner TM1 domains, determines the activation threshold of the channel33. Correspondingly, it was found that by decreasing the hydrophobicity of the gate, the tension threshold could be lowered22. This property of MscL made possible the design of various controllable valves34, primarily for drug delivery purposes. For all the aforementioned properties and based on its fundamental role of translating cell membrane excessive tensions into electrophysiological activities, MscL makes a great fit as a mechanoelectrical transducer in DIBs.
In this article, we present an original micropipette-based method to form DIBs and measure the activity of the incorporated MscL channels under mechanical stimulation. We report for the first time, the response of DIBs to mechanical stimulus and the functional reconstitution of the V23T low-threshold mutant of MscL in DIBs35.
The experimental system consists of lipid encased aqueous droplets anchored to the tips of two opposing borosilicate glass micropipettes. When droplets are brought into contact a lipid bilayer interface is formed. This technique offers control over the chemical composition and size of each droplet (bulk), as well as the dimensions of the bilayer interface. In addition, asymmetric membranes with various lipid compositions in each leaflet could be easily formed. Having one of the micropipettes attached to a harmonic piezoelectric actuator, provides the ability to apply a pre-programmed single-cycle or oscillatory stimulus. Tension is delivered to the artificial membrane through the compression of both droplets supporting it. As a result of droplet deformation, the areas of water-lipid-oil interfaces increase, and simultaneously the angle between the droplets decreases, causing an increase in membrane tension and transient MscL activation. Through analysis of the shapes of the droplets during deformation, the tension created at the interface could be estimated. Even though the focus in this article is on the mechano-transduction properties of the DIB, we also emphasize that other types of biomolecules, such as alamethicin, can be activated by this multi-functional platform. We present here, all the technical aspects of preparing, assembling, and taking measurements with this new method in a step-by-step manner.
Protocol
1. Preparation of PEG-DMA Hydrogels
- Select an appropriate measuring/mixing container (flask, beaker, etc.) for the application. Clean it thoroughly using detergent and water, and then wipe it with lint-free tissue wipers.
- Wear gloves to avoid contaminating the glassware with oils from fingertips. Rinse the container with enough deionized water to remove detergent residue.
- Wipe the container with lint-free tissue to get rid of the water, then spray with isopropyl alcohol (IPA, 99.5%) and wipe until clean. Place it in a vacuum chamber to allow all IPA to completely evaporate. Clean the rest of the lab equipment used in the hydrogel forming process with distilled water.
- To prepare a 40% (w/v) PEG-DMA hydrogel solution, weigh 4 g of the poly(ethylene-glycol) dimethacrylate (PEG-DMA; MW = 1,000 g/mol) polymer using a laboratory scale.
- Place the weighed PEG-DMA into flask and heat using a sonicator bath at 45-55 °C until the solid PEG-DMA has liquefied. During the process, cover the opening of the flask with Parafilm/wax paper to keep water out.
- Once the PEG-DMA has liquefied, add buffer solution (500 mM KCl, 10 mM MOPS, pH 7.0) until total volume reaches ~10 ml (enough for several experiments over a six months period).
- Add the curing agent at 0.5% (w/v). In this case, add 0.05 g of the curing agent to the 10 ml mixture. Place the flask back into the sonicator bath and allow the components to dissolve in solution (around 10 min, 250 watts).
NOTE: Once the curing agent has been added to the solution, the hydrogels will cure (solidify) if exposed to any light source for a sufficient amount of time. To help combat this, wrap the vial/container with black tape and store it in a dark place. This solution can be stored for several weeks at room temperature (22 °C).
2. Preparation of Liposomes
- Prepare 10 ml of a 2 mg/ml lipid solution by adding 10 ml of buffer (500 mM KCl, 10 mM MOPS, pH 7.0) to 20 mg of 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC) synthetic lipids purchased as lyophilized powder. Make sure both lipid vesicles and buffer solution are thoroughly mixed (the mixture should look homogenous and hazy when everything is dissolved).
- Freeze (-20 °C) and completely thaw the new lipid mixture for a total of six times. Let the mixture thaw at room temperature, never in a heated environment.
- Using a commercially available extruder, extrude the lipids by forcing the whole lipid suspension first through a 0.4 μm polycarbonate membrane filter and then six times through a 0.1 μm membrane filter. This process yields particles with diameters near 100 nm (equal to the pore size of the filter).
NOTE: Other lipids and lipid ratios can be prepared using this method. Liposomes should be stored at 4 °C for several weeks.
3. MscL Isolation and Reconstitution
- Streak out a temperature agarose plate, containing 100 μg/ml ampicillin, E. coli MJF465 cells with a pB10b plasmid carrying the V23T MscL gene extended with a 6-His tag on the 3' end (C-terminus). Allow the plate to culture overnight (12-16 hr) at 37 °C in a stationary incubator. The plasmid is selected for and retained in cells with 100 μg/ml ampicillin in standard LB medium. The next day place 20 ml of LB media with 100 μg/ml ampicillin in a culturing vesicle (a 50 ml flask or what is available to hold the culture). Take the plate that was cultured overnight and select a colony from the plate to transfer (inoculate) to the prepared 20 ml LB media with a sterile inoculation stick. Allow the 20 ml culture to grow overnight (12-16 hr) at 37 °C at 250 rpm in a shaking incubator.
- Decant the 20 ml overnight culture into 2-4 L of LB medium. Ampicillin is no longer required. Shake the flasks in a shaking incubator at 250 rpm at 37 °C until OD600 reaches 0.5. Add Isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 0.6 mM and let the culture go for another hour (to OD600 = 0.8-1.0).
- Put the flasks on ice to chill the cultures and then collect the bacteria by centrifugation. Use six 400 ml conical tubes (or as many as allowed by the rotor used) and centrifuge for 5-8 min at 7,438 x g which is enough to pellet the bacteria. Decant the supernatant, and repeat the procedure until all the cells from the media are harvested. The number of spins required varies based on the amount of culture that has been grown and the rotor used. For a 2 L culture it is only needed to spin down the cells once. Transfer all harvested cells into a single centrifuge tube.
- Resuspend the cell pellets in ~20 ml of French press buffer (100 mM KPi and 5 mM MgCl2, pH 7.4). The suspension should be dense (like cream or milk). Immediately before French-pressing the suspension add the protease inhibitor phenylmethylsulfonyl fluoride (PMSF) to a final concentration of 2 mM and mix vigorously.
- French-press the culture, in a 35 ml French-pressure cell, at 10,000 to 16,000 psi. Spin down the suspension to separate out the unbroken cells at 7,438 x g, 10 min at 4 °C.
- Put the supernatant in a separate tube, and add to it lysozyme and DNase (0.2 mg/ml each). Let supernatant tumble for 10 min at room temperature.
NOTE: DNase is optional; it reduces the viscosity for the high-speed centrifugation. Lysozyme is critical; it digests the remnants of cell wall and helps increase the yield of the membrane extraction done with a mild non-denaturing detergent.
- Distribute the supernatant mix into two ultracentrifuge tubes and spin them at 106,883-153,911 x g (depending on the rotor) at 4 °C for 40 min. After centrifugation the supernatant is decanted and the brownish pellet at the bottom (the total membrane fraction) can be frozen in the tube for long-term storage (- 80 °C) or used for immediate protein purification.
- Prepare 0.5-1 L of High Imidazole buffer: 100 mM NaCl + 500 mM imidazole, titrate to pH 7.2-7.4 with concentrated HCl. Note that imidazole is a good buffering substance by itself.
- Prepare 0.5-1 L of Low Imidazole buffer: 100 mM NaCl, + 15 mM imidazole, by appropriately diluting the buffer above with 100 mM NaCl. No pH adjustment is required.
- Take 100-150 ml of each buffer in separate bottles, and add 1% (w/v) b-octyl glucopyranoside (OG). Stir the solution well and filter it through a 0.22 μm filter. These solutions are the low- and high-imidazole chromatography solutions.
- Prepare Extraction buffer, take 50 ml of Low-imidazole buffer, and add 3% (w/v) OG and filter the buffer.
- Use membrane pellets of 0.5-2 g wet weight for protein isolation. Add 5-7 ml of extraction buffer, resuspend the pellet, and homogenize it in a 30 ml hand-driven glass-piston homogenizer. With 5-10 gentle strokes make a homogeneous suspension without lumps. Use caution, shear stress is known to cause protein denaturation.
- Spin down the insoluble particles (mid-range centrifuge, fixed-angle rotor, 38,478-68,405 x g, at 4 °C for 15 min). Meanwhile, take 3 ml of Ni NTA beads (6 ml of suspension) and wash them once with the low-imidazole buffer (w/o OG) by shaking them in a 15 ml screw-cap tube. Let the beads settle on the bottom (~ 5-7 min) or spin them down at 129-201 x g for one minute at 4 °C. Once the pellet is formed, carefully decant the supernatant by hand and repeat the procedure. Equilibrate the beads with 2-3 ml of 3% OG extraction buffer.
- Mix the homogenized mixture (membrane pellet and extraction buffer) from 3.12 with 3-3.5 ml Ni NTA beads. Let the mix tumble in a screw-cap tube for 60 min (batch-loading). Spin the beads down at 201 x g (30 sec), decant the supernatant by hand, and wash the beads with 1% OG low-imidazole buffer once. Pellet the beads as in 3.13 again and resuspend them in 20-30 ml of fresh low-imidazole buffer.
- Pack a small column (equipped with an upper flow adapter) with the Ni NTA beads, and let the beads settle by opening the stopcock to let the extract flow through (do not allow the beads to dry). Wash the beads with one aliquot of 10 ml low-imidazole buffer (1% OG) with the stop cock open.
- Load the chromatography machine with pure low- and high-imidazole buffers about 25 ml of each at the machine defined flow rate (varies per machine); this is done by passing the low-imidazole buffer through the system first. Zero the optical recorder at OD260 (baseline). Note that the buffer is quite different from water because low-grade imidazole has impurities which absorb UV. Insert the flow adapter to the column and attach it to the machine.
- Wash the column again with low-imidazole buffer (at 1 ml/min) until the OD of the flow through comes reaches the baseline (it may take 10-20 ml). This removes the unbound proteins from the column.
- Apply a linear gradient of imidazole of 20 to 500 mM, for 30 min, at 1 ml/min. Start collecting 4 ml fractions when OD600 shows an increase. The first two fractions are full of loosely bound proteins, whereas MscL-6His starts elution at ~40% of the linear gradient. The majority of the protein appears in fractions 3 to 8.
NOTE: a linear rise of OD will be observed due to the increasing % of imidazole. - Pool the fractions 3-4, 5-6, and 7-8 together. Optionally, concentrate the fractions individually. Concentrate the fractions 6-10 fold using centrifugal filters. After a 20 min spin at 804 x g and at 4 °C, carefully resuspend the concentrated protein, the protein tends to stick to the filter.
- Withdraw 50 μl aliquots, mix them with SDS sample buffer, and check for protein purity using PAGE gel electrophoresis.
NOTE: MscL will migrate as a fuzzy band of about 17 kDa to the bottom of the gel. - Use the concentrated fractions to quantify the protein using a protein assay kit, following the manufacturer's instruction. A typical yield from a 0.8 g membrane pellet is up to 0.2 mg of pure protein in the combined fractions.
- Reconstitute V23T MscL into DPhPC liposomes through dialysis. Take a 10 mg/ml chloroform solution of DPhPC and aliquot 0.5 ml (i.e. 5 mg of lipid) of it into three disposable round-bottom (12 x 130 mm) glass tubes. Dry the lipid under the stream of nitrogen and remove the remnants of chloroform under vacuum (4-6 hr).
- Add 15 20 mg of powdered OG to the dry lipid in each tube, dissolve it in 2 ml of dialysis buffer (100 mM KCl, 5 mM KPi, pH 7.2), vortex, and mildly sonicate. The OG-solubilized lipids should form a clear solution.
- Add concentrated V23T MscL solutions to each tube to achieve 1:100, 1:300 and 1:1,000 protein-to-lipid ratios and vortex well. Cut and wash three pieces (~12 cm long) of dialysis tubing (MWCO 8000, 7.5 mm diameter, have three pairs of numbered clips ready.)
- Place the solubilized lipid-protein mixtures inside the tubing, carefully close the ends with clips, and dialyze against 2 L of buffer (100 mM KCl, 5 mM KPi, pH7.2) for 48 hr at 4 °C with four changes of the buffer every 12 hr. After dialysis, the proteo-liposomes are ready.
NOTE: The liposome solution can be supplemented with 2 mM of NaN3 (sodium azide) and stored at 4 °C. Avoid freezing.
4. Manufacture of the Oil Reservoir
- Drill two opposing holes (1.0 mm in diameter) all the way through the wall of a 2 inch diameter, 1.5 cm long acrylic cylinder at 1 cm from the bottom (Figure 1A).
- Drill two 4 mm holes concentric to the previously drilled 1 mm holes. The depths of the holes should be 1 mm each (make sure not to drill all the way). These holes are made to fit the rubber gaskets.
- Place and glue two rubber gaskets having 1 mm inner diameters in the bigger holes in order to prevent oil from leaking.
- Glue the machined cylinder to a 10 cm x 10 cm thin acrylic sheet using any multipurpose epoxy (Figure 1).
On the day of the experiment:
5. Preparation of Electrodes
- Cut a 7 cm length of two 250 μm-diameter silver wires, and then immerse their tips in bleach for two hours to form a silver-chloride (AgCl) coating. A gray color indicates that an AgCl coating has been formed (Figure 2E).
- Using a glass cutter, split a 10 cm long, 1/0.58 OD/ID mm borosilicate class capillary into two 5 cm capillaries.
- Using a 34 gauge microfil needle fill the capillaries with the PEG-DMA hydrogel. To prevent the hydrated hydrogel from swelling out of the capillary, keep a 3 mm clearance at the tips and make sure there are no air bubbles in the capillaries.
- Insert the Ag/AgCl electrodes into the hydrogel filled capillaries (Figure 2E).
- Cure the PEG-DMA hydrogel through free-radical photopolymerization upon exposure to UV light for 2 min at 1 W using a UV spot gun.
6. Setting Up the Experiment
NOTE: The experiment is setup under a faraday cage grounded to a ground connection on the patch amplifier.
- Attach one of the micropipettes to a straight microelectrode holder that has a male connector (Figure 2E).
- Connect the microelectrode holder to the headstage of the patch amplifier (Figure 2A). To connect the headstage to ground, solder an 18 gauge insulated copper wire to an appropriate connector for the headstage.
- Mount the headstage on a 3-axis manual micromanipulator. Attach the headstage mounting plate (it should be bought along with the micromanipulator or custom made in a machine shop) to the micromanipulator and then connect the headstage to the mounting plate using appropriate screws.
- Attach the second micropipette to the linear actuator through a lab-made connector and then mount both on a second micromanipulator (Figure 2B). The micropipettes should be opposing each other, aligned, and horizontally leveled. Note: the brand and style of the manipulators do not matter.
- In a glass vial, mix 0.1 ml of the DPhPC liposomes with 0.01 ml of the V23T MscL proteoliposome solution.
NOTE: This step is needed to reduce the protein-to-lipid ratio (~0.0002), which is critical to the formation of a stable lipid bilayer.
- Using a 34 gauge microfil needle, fill the tips of both micropipettes with proteoliposome solution (Figure 3A).
- Place reservoir on top of an upright microscope, and feed the micropipettes through the opposing 1 mm holes (Figure 2B). NOTE: any microscope could be used as long at the droplets could be clearly seen.
- Fill reservoir to the surface with Hexadecane (99%). The Hexadecane does not need further purification.
- To form the spherical droplets at the tip of the micropipettes, use a third 10 μm-diameter borosilicate glass micropipette, mounted on a third micromanipulator, to dispense diluted V23T MscL proteoliposome solution (~0.00052 ml) at the tips of the micropipettes and form the droplets (Figure 3).
- Control the size of the droplets (by decreasing or increasing the volume) as desired and let them rest for 10 min for the monolayers to form completely (Figure 3).
- Bring the droplets into contact, bilayer formation will occur within 1 to 2 min.
7. Setting Up the Software and Equipment
- Prepare the software by turning on the computers, microscope, piezoelectric oscillator controller, function generators, patch amplifier, and the low-noise data acquisition system.
NOTE: any patch amplifier could be used and the following instructions are specifically for the one we used and which is listed in the materials and equipment list. - On the front panel of the patch amplifier, set the "Mode" knobs to VHOLD/IHOLD and V-CLAMP.
- On the front panel set the "Lowpass" Bessel Filter to 1 kHz and Output Gain to 2.
- Set the "Configuration" to WHOLE CELL β = 1.
- Make sure the rest of the knobs are set to zero or in neutral position.
- Sample all DIB current measurements at 5 kHz with a 1 kHz Bessel anti-aliasing filter.
- Run software by double-clicking on the icon on the desktop.
- Click "Configure > Digitizer" to open the "Digitizer" dialog, and then click the "Change" button.
- In the "Change Digitizer" dialog select "Digidata 1440 Series" from the "Digitizer Type" list.
- Click the Scan button to detect the digitizer.
- Click "OK" to exit the "Change Digitizer" dialog, and then click "OK" to exit the "Digitizer" dialog.
- Click "Configure > Lab Bench".
- In the Input Signals tab of the lab Bench, select Analog In Å under Digitizer Channels. Set the scale factor to 0.002.
8. Formation of the Lipid Bilayer
- Using a BNC cable, connect the output of a waveform generator to the external command input front switched (on the rear panel of the data acquisition system). Send a 10 Hz, 500 mV pk-to-pk triangular waveform to the headstage.
- Using the micromanipulator, move the glass micropipettes horizontally to bring droplets into contact until they slightly touch and wait for bilayer thinning to occur (usually around 1 to 2 min) (Figures 3C and 3D).
NOTE: Progression of the bilayer formation process can be seen visually through the microscope and may be monitored by current measurement (Figure 4).
- Adjust the bilayer size (~250 μm in diameter) by controlling the position of the droplet mounted on the actuator, using the micromanipulator. Note: the bilayer size could be estimated visually through the microscope. This method makes it easier for the researcher to control the size of the bilayer by easily moving the droplets using the micromanipulators.
9. Dynamic Excitation and MscL Gating
- Once the bilayer has formed and is stable (i.e. the bilayer is not breaking or conductive), stimulate the droplets by sending a sinusoidal signal using a function generator.
- To stimulate the MscL protein incorporated in the bilayer, send a sinusoidal waveform with a 175 μm peak-to-peak amplitude, 0.2 Hz frequency, and 50% duty cycle to the piezoelectric servo-controller. (Various types of waveforms could be sent with different amplitudes, frequencies, and duty cycle)
10. Processing and Interpreting Results
- Save the current measurements, recorded using the data acquisition system, in .ABF format. Import data (in .ABF format) to Matlab using a function file "abfload", then analyze and process the data. The "abfload" file is available for free online.
- Estimate the tension in the bilayer and areal expansion of the droplets, using videos of the droplet during full actuation cycles that are recorded using an appropriate camera.
- Process videos in Matlab, by processing individual frames using image processing techniques to estimate the area of the water/oil interface, as well as the angle between the droplets. NOTE: using a 2D frame taken from the video, detect the water-oil interface (i.e. the edge of the droplet) and then estimate the surface area by revolution.
Representative Results
Figures 1 and 2 display the experimental setup and equipment used to record protein activity in the course of mechanical stimulation of the lipid bilayer membrane. To minimize electrical noise into our measurements, the workstation is placed within a lab-made Faraday cage, grounded to a ground connection on the AxoPatch 200 B Amplifier.
Formation of a stable insulating lipid bilayer is a key step in this study. In this arrangement, a lipid monolayer assembles at the oil/water interface of the aqueous droplets immersed in a bath of an organic solvent. When droplets are placed in contact, excess oil is eliminated, and the opposing lipid monolayers thin to a two-molecule thick lipid bilayer. The most common technique used in bilayer characterization is voltage-clamp. With voltage-clamp, the voltage across the bilayer is maintained at a constant value while the current is measured. Figure 4 portrays a typical real-time current recording of the initial bilayer formation. Knowing the specific capacitance (~0.6 μF/cm2)5 of the DPhPC lipid bilayer, the area of the formed bilayer could be calculated. The bilayer area could be controlled by changing the position of the droplets (Figure 4A). Using the piezoelectric actuator, different types of waveforms (sinusoidal, square, triangular, etc.) at different frequencies, amplitudes, and duty cycles could be applied to the droplets to horizontally and axially oscillate them and thus, bilayer tension and area could be altered (Figure 4B).
When the DIB is mechanically stimulated, while maintaining a constant DC potential across the membrane, a low-threshold (gain-of-function) V23T mutant of MscL generates reliable activities including mainly sub-conductive states and occasionally full opening events (Figure 5). These events are identical to those recorded using the patch-clamp technique from intact inner E. coli membranes and liposomes reconstituted with the purified V23T MscL. The results in Figure 5 prove that gating occurs in response to an increase in tension, since all current spikes are observed at peak compression. At peak compression, the relative areal expansion of the droplets is maximal and therefore, tension at the interface is maximal.
Alamethicin, a voltage-gated ion channel and one of the most studied peptides, increases the membrane permeability when a DC voltage is applied across the membrane36. The ability of the lipid bilayer interface to host transmembrane proteins and peptides is also tested by performing voltage-gating current recordings using alamethicin peptide. Alamethicin is mixed with the phospholipid solution to a final concentration of 100 ng/ml. Figure 6 shows the current measurements under voltage clamp (+115 mV). The droplets in this experiment are pulled apart in order to achieve small bilayer interface and thus higher resistance and smaller capacitance. The gating behavior of the Alamethicin peptide is shown through the discrete steps of current (Figure 6). The histogram on the right side of the plot shows the changes in conductance from the base level (0.0962 nS), which is basically the first conductance level of the channel itself.

Figure 1: A schematic describing the main parts and dimensions of the oil reservoir. The oil reservoir is manufactured at the machine shop at Virginia Tech. It consists of a machined cylindrical acrylic tube glued to the surface of an acrylic sheet. The dimensions and design can be modified to accommodate different applications or more than two micropipettes. Please click here to view a larger version of this figure.
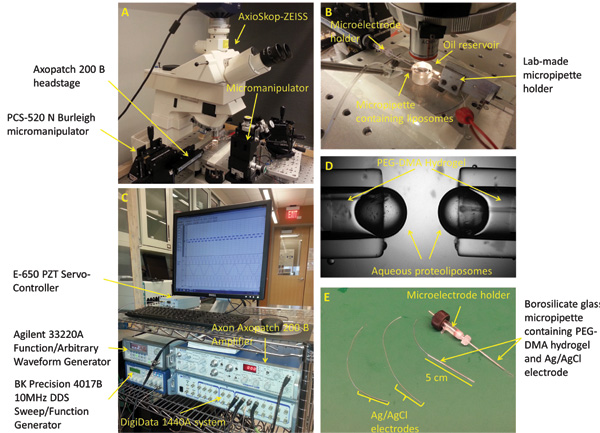
Figure 2: Experimental setup and micropipettes preparation. (A) The standard workstation for forming, mechanically stimulating, and characterizing the interface bilayers includes a microscope, 3-axis manipulators, a digital camera, piezoelectric oscillator, vibration isolation table, and a Faraday cage (not shown). (B) The experimental setup consists of two opposing PEG-DMA hydrogel filled micropipettes horizontally positioned within a bath of Hexadecane oil. Each of the micropipettes contains an Ag/AgCl electrode to provide electrical connection. A third micropipette filled with proteoliposome solution is used to form the droplets at the tip of the other micropipettes. (C) The DIB current response could be measured using a combination of the patch amplifier and the low-noise data acquisition system. (D) A closed up picture showing the aqueous droplets formed at the tip of the micropipettes. (E) Ag/AgCl electrodes are made by dipping the tip of two 250 μm silver wires in bleach. The electrodes are then fed through two borosilicate glass capillaries filled with PEG-DMA hydrogel, which is cured with UV light to solidify. A straight microelectrode holder with male connector is used to connect one of the micropipettes to the headstage of the patch amplifier.
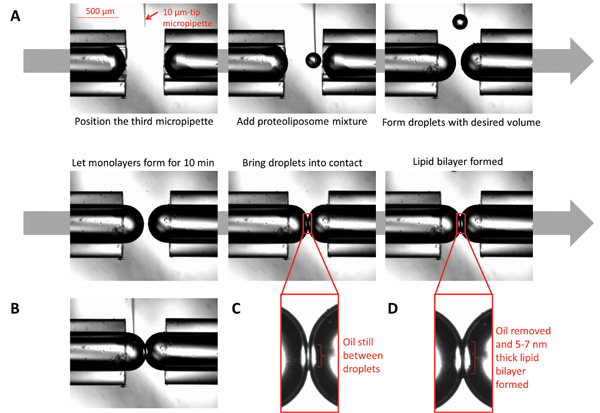
Figure 3: Images illustrating the formation of droplet interface bilayers. (A) A 10 μm micropipette filled with proteoliposomes is positioned under the microscope in proximity to the micropipette tips. Using a syringe connected to the micropipette, dispense small volumes of the proteoliposomes to form spherical droplets to desired volume. Let the monolayer form by allowing the droplets to sit for ten min. Bring the droplets into contact; the bilayer will form after all oil at the interface is eliminated. (B) While the bilayer is formed, the chemical composition at both sides of the interface could be controlled by injecting desired chemicals using a micro-sized micropipette. (C) The droplets at the moment of first contact. (D) The droplets when the lipid bilayer is formed.
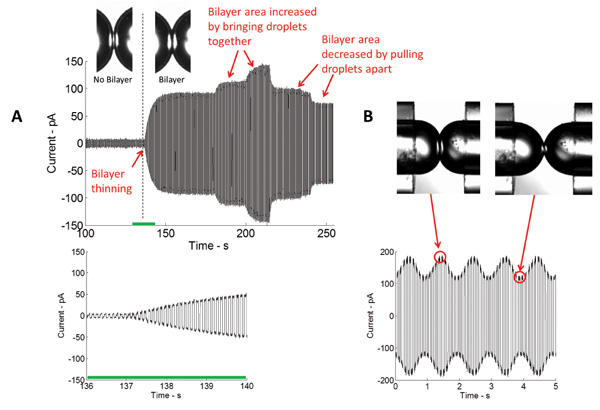
Figure 4: Real-time measurements show both the initial thinning and subsequent expansion of the interface. (A) Current measured in the course of bilayer formation through the application of a triangular electrical potential. The magnitude of the measured current is directly proportional to the capacitance, and thus the area of the bilayer interface. The closer the droplets are brought together, the bigger the area of the interface and vice versa. (B) Upon application of mechanical excitation, the area of the bilayer interface increases and decreases at the same frequency as the stimulating signal.
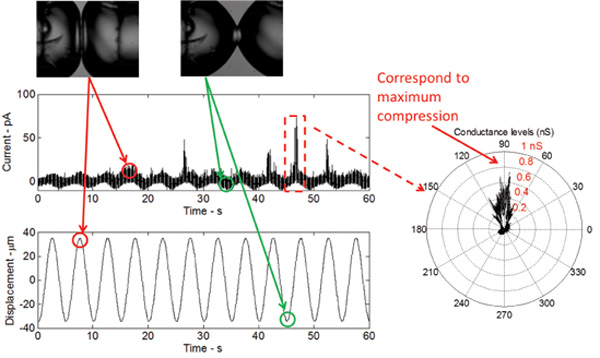
Figure 5: Real-time measurements show the response of the bilayer to mechanical excitation as well as the gating of the V23T mutant of MscL. The shape of the current response is sinusoidal, which relates to a sinusoidal change in bilayer capacitance as a result of the bilayer area change. The current spikes, occurring at the peak of each cycle, indicate sub-conductance gating of the V23T mutant. A polar plot further indicates that gating occurs at peak compression, which reflects an increase in tension at the bilayer interface.
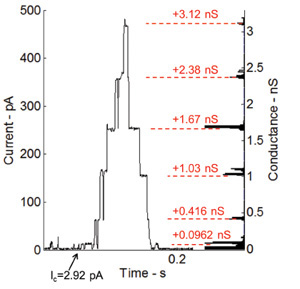
Figure 6: Current measurements under voltage clamp and corresponding histogram of conductance levels for gating activity of incorporated Alamethicin channels. The gating behavior of the Alamethicin peptide is shown through the discrete step-wise increase in current. The conductance levels match very well with previous measurements performed by our research group at Virginia Tech7.
Discussion
Mechanosensation signifies one of the first sensory transduction pathways that evolved in living organisms. Using this phenomenon for studying and understanding the mechano-electrical properties of the DIB, is a crucial step toward functional stimuli-responsive materials. It involves the incorporation and activation of a mechanosensitive channel, MscL, in the DIB as a mechanoelectrical transducer and a strain gauge to detect tension increase in the lipid bilayer interface. On another note, the function of MS channels could be regulated through the basic material properties of lipid bilayers including thickness, intrinsic curvature, and compressibility. In light of the aforementioned, the micropipette-based technique provides a valuable tool allowing the researcher the ability to study MS channels in DIBs and provides insights into the structure of the lipid bilayer, as well as the lipid-protein interactions.
Over the past three decades, patch-clamp was the primary method to study MS channels, since it allows clamping of both voltage and tension. However, patch-clamp requires bulky equipment and not suitable for miniaturization, a property required for the engineering of sensory and conversion devices. DIBs due to their simplicity, stability, and compactness represent a suitable environment to study the activity of MscL. Here, we extend previous advances in the DIB formation techniques by proposing a micropipette-based technique, with the ability to control the size of droplets and bilayer interface, the chemical composition of each droplet, and the tension at the interface through dynamic stimulation. The technique consists of anchoring aqueous droplets, containing proteoliposomes, to the tips of coaxially opposing glass capillaries. The droplets are placed in a bath of organic solvent and when brought in contact a lipid bilayer forms at the interface.
The micropipettes are attached to piezoelectric oscillators, allowing horizontal displacement of the droplets. Dynamically compressing the droplets, results in an increase of interfacial tension at the water oil interface and therefore an increase in bilayer tension. Two major aspects differentiate this method from the similar and recently published contact bubble bilayer (CBB) technique37. Using the technique presented herein, the size of the bilayer is controlled using micromanipulators and thus the volumes of the droplets remain constant, unlike in the CBB method. In addition, the CBB technique calls for pressure pumps, which are not needed in the method presented in this paper making it simpler and easier to build.
We are able to incorporate and stimulate bacterial MscL for the first time without the use of a patch pipette or chemical modifications38. Since the system facilitates the formation of robust asymmetric lipid bilayer membranes, it more closely mimics the lipid asymmetry found in biological membranes. This allows us to study the effects of controlled membrane composition or asymmetry on the activity of MscL. Additionally, through image processing techniques, this method helps estimate the tension at the bilayer interface. This technique assists in understanding the principles of interconversion between bulk and surface forces in the DIB, facilitates the measurements of fundamental membrane properties, and improves the understanding of MscL response to membrane tension.
Although this method takes us a step closer toward a biomolecular stimuli-responsive material system and to a different physiological environment to study MscL, there are limitations to the system. Tension in this system cannot be clamped due to the presence of the lipid reservoir in the form of liposomes in each droplet, which tends to relieve tension at the oil/water interface. Therefore, at present mechanosensitive channels can be stimulated in DIBs only in a dynamic regime. The presence of air bubbles in the system significantly affects the precision and reproducibility of the experiments. Air bubbles present in the hydrogels could result loss if electrical connection.
While we describe the use of the micro-pipette based method for the stimulation of MscL, the technique could be used to study other types of MS channels and has the potential to be used by researchers to study a variety of biomolecules. For instance, similar setup has been used in our lab to study the mechanoelectrical response of a channel-free droplet interface bilayer membrane. Various proteins could be reconstituted and activated using this highly controlled setup, taking in consideration that the reconstitution environments of each biomolecule vary. The method described in this article touches on a considerably wider application potential that is only limited to the imagination of the researcher.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
Research reported in this publication is supported by the Air Force Office of Scientific Research Basic Initiative Grant FA9550-12-1-0464.
Materials
| 0.22 µm filter | Corning | 430624 | |
| 1,2-diphytanoy-sn-glycero-3-phosphocholine (DPhPC) | Avanti Polar Lipids | 850356P | Purchased as lyophilized powder |
| 34-gauge microfil | World Precision Instruments | MF24G-5 | |
| 400 mL Centrifuge bottels | ThermoFisher | 3141 | Nalgene |
| Agilent Function/Arbitrary Waveform Generator, 20 MHz | Keysight Technologies | 33220A | |
| Ampicillian | ThermoFisher | BP1760 | ACS Grade |
| Avanti® Mini-Extruder | Avanti Polar Lipids | 610000 | |
| Axio Scope.A1 | Carl Zeiss | – | |
| AxioCam HSm | Carl Zeiss | – | |
| Axopatch 200B Amplifier | Molecular Devices | – | |
| BCA protein assay kit | Pierce | 23225 | |
| BK Precision 4017B 10 MHz DDs Sweep/Function Generator | Digi-Key | BK4017B-ND | |
| Borosilicate Glass Capillaries | World Precision Instruments | 1B100F-4 | |
| Dialysis tubing | 7 Spectra/Por | 132113 | MWCO 8000, 7.5 mm diameter |
| DigiData 1440A system | Molecular Devices | – | |
| DNAse | Sigma-Aldrich | DN25 | |
| DPhPC | Avanti | 850356C | |
| E-625 PZT Servo-Controller | Physik Instrumente | E-526 | |
| FPLC System | Pharmacia Biotech | – | |
| HCl | J.T. Baker | 9535-33 | |
| Hexadecane, 99% | Sigma-Aldrich | 544-76-3 | |
| Homoginizer | Wheaton | 357426 | 15 mL |
| Imidazole | Sigma-Aldrich | I5513 | |
| IPTG | Affymetrix | 17886 | |
| IRGACURE® 2959 | IRGACURE® | 555047962 | |
| Isopore Membrane Filters | EMD Millipore | VCTP02500 | |
| Isopropyl Alcohol | VWR International | BDH1133-4LP | |
| KCl | Sigma-Aldrich | P3911 | ACS Grade |
| KH2PO4 | Mallinckrodt | 7100 | ACS Grade |
| Kimble-Chase | Kontes | 420401-1515 | Flex-Column |
| LED-100 UV Spot Curing System | Electro-Lite, corp. | 81170 | |
| Lysozyme | Sigma-Aldrich | L6876 | |
| Manual Patch-Clamp Micromanipulators | Thorlabs | PCS-520N | |
| MgCl2 | ThermoFisher | M33 | ACS Grade |
| Microelectrode Holder | World Precision Instruments | MEH1S | |
| Micropipette Puller | Sutter Instruments | P-1000 | |
| MOPS, minimum 99.5% titration | Sigma-Aldrich | M1254-100G | |
| N2 Gas | Airgas | UN1066 | |
| NaCl | EMD | SX0420-1 | ACS Grade |
| Ni NTA agarose beads | Qiagen | 1000632 | |
| Optically Clear Cast Acrylic Tube, 2-1/2" OD x 2" ID | McMaster-Carr | 8486K545 | |
| P-601 PiezoMove Flexure-Guided Linear Actuator | Physik Instrumente | P-601 | |
| PAGE gel | Bio-Rad | 456-9033 | |
| Parafilm M® All-Purpose Laboratory Film | Parafilm® | PM999 | |
| Phenylmethylsulfonyl fluoride | Sigma-Aldrich | P7626 | |
| Poly(ethylene glycol)1000 dimethacrylate | Polysciences, Inc. | 15178-100 | |
| Polycarbonate (PCTE) Membrane Filters, Black, 0.4 Micron, 25mm, 100/Pk | Sterlitech Corporation | PCTB0425100 | |
| Potassium Chloride | Sigma-Aldrich | P5405-500G | |
| Powder Free Soft Nitrile Examination Gloves | VWR International | CA89-38-272 | |
| Replacement Gasket 1.0mm | World Precision Instruments | GO1-100 | |
| SDS | Sigma-Aldrich | L5750 | |
| Silver wire | GoodFellow | 147-346-94 | Different diameters could be used depending on the application |
| Sodium Azide | Affymetrix | 21610 | |
| Test tubes | ThermoFisher | 14-961-27 | 12 x 130 mm |
| Tryptone | ThermoFisher | BP1421 | |
| Ultracal 30K | Millipore | UFC803024 | Amicore Ultra 30 MWCO |
| VWR Light-Duty Tissue Wipers | VWR International | 82003-820 | |
| VWR Scientific 50D Ultrasonic Cleaner | VWR International | 13089 | |
| Water Purifier | Barnstead | D11931 | |
| Yeast | ThermoFisher | BP1422 | |
| β-octylglucopyranoside | Anatrace | O311S |
Referenzen
- Montal, M., Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. P NATL ACAD SCI USA. 69, 3561-3566 (1972).
- Tsofina, L., Liberman, E., Babakov, A. Production of bimolecular protein-lipid membranes in aqueous solution. Nature. , (1966).
- Funakoshi, K., Suzuki, H., Takeuchi, S. Lipid Bilayer Formation by Contacting Monolayers in a Microfluidic Device for Membrane Protein Analysis. ANAL CHEM. 78, 8169-8174 (2006).
- Holden, M. A., Needham, D., Bayley, H. Functional bionetworks from nanoliter water droplets. J AM CHEM SOC. 129, 8650-8655 (2007).
- Hwang, W. L., Chen, M., Cronin, B., Holden, M. A., Bayley, H. Asymmetric droplet interface bilayers. J AM CHEM SOC. 130, 5878-5879 (2008).
- Maglia, G., et al. Droplet networks with incorporated protein diodes show collective properties. Nat Nano. 4, 437-440 (2009).
- Sarles, S. A., Stiltner, L. J., Williams, C. B., Leo, D. J. Bilayer formation between lipid-encased hydrogels contained in solid substrates. ACS APPL MATER INTER. 2, 3654-3663 (2010).
- Sarles, S. A., Leo, D. J. Regulated attachment method for reconstituting lipid bilayers of prescribed size within flexible substrates. ANAL CHEM. 82, 959-966 (2010).
- Sarles, S. A. . Physical Encapsulation of Interface Bilayers. , (2010).
- Sarles, S. A., Leo, D. J. Membrane-based biomolecular smart materials. SMART MATER STRUCT. 20, 094018 (2011).
- Sarles, S. A. The use of virtual ground to control transmembrane voltages and measure bilayer currents in serial arrays of droplet interface bilayers. SMART MATER STRUCT. 22, 094023 (2013).
- Sarles, S. A., Leo, D. J. Cell-inspired electroactive polymer materials incorporating biomolecular materials. SPIE Smart Structures and Materials+ Nondestructive Evaluation and Health Monitoring. , 797626 (2011).
- Hwang, W. L., Chen, M., Cronin, B., Holden, M. A., Bayley, H. Asymmetric droplet interface bilayers. Journal of the American Chemical Society. 130, 5878-5879 (2008).
- Bayley, H., et al. Droplet interface bilayers. MOL BIOSYST. 4, 1191-1208 (2008).
- White, S. H. Analysis of the torus surrounding planar lipid bilayer membranes. BIOPHYS J. 12, 432 (1972).
- Tien, H. T., Ottova, A. L. The lipid bilayer concept and its experimental realization: from soap bubbles, kitchen sink, to bilayer lipid membranes. J MEMBRANE SCI. 189, 83-117 (2001).
- Perozo, E. Gating prokaryotic mechanosensitive channels. NAT REV MOL CELL BIO. 7, 109-119 (2006).
- Kung, C., Martinac, B., Sukharev, S. Mechanosensitive channels in microbes. ANNU REV MICROBIOL. 64, 313-329 (2010).
- Bialecka-Fornal, M., Lee, H. J., DeBerg, H. A., Gandhi, C. S., Phillips, R. Single-cell census of mechanosensitive channels in living bacteria. PLoS ONE. 7, e33077 (2012).
- Levina, N., et al. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. The EMBO Journal. 18, 1730-1737 (1999).
- Chiang, C. -. S., Anishkin, A., Sukharev, S. Gating of the large mechanosensitive channel in situ: estimation of the spatial scale of the transition from channel population responses. BIOPHYS J. 86, 2846-2861 (2004).
- Anishkin, A., Chiang, C. -. S., Sukharev, S. Gain-of-function mutations reveal expanded intermediate states and a sequential action of two gates in MscL. J GEN PHYSIOL. 125, 155-170 (2005).
- Sukharev, S. I., Sigurdson, W. J., Kung, C., Sachs, F. Energetic and Spatial Parameters for Gating of the Bacterial Large Conductance Mechanosensitive Channel, MscL. J GEN PHYSIOL. 113, 525-540 (1999).
- Deplazes, E., Louhivuori, M., Jayatilaka, D., Marrink, S. J., Corry, B. Structural investigation of MscL gating using experimental data and coarse grained MD simulations. PLOS COMPUT BIOL. 8, e1002683 (2012).
- Kubalski, A., Martinac, B. . Bacterial ion channels and their eukaryotic homologs. , (2005).
- Chang, G., Spencer, R. H., Lee, A. T., Barclay, M. T., Rees, D. C. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 282, 2220-2226 (1998).
- Steinbacher, S., Bass, R., Strop, P., Rees, D. C. Structures of the prokaryotic mechanosensitive channels MscL and MscS. Mechanosensitive Ion Channels, Part A. , 1-24 (2007).
- Sukharev, S., Durell, S. R., Guy, H. R. Structural models of the MscL gating mechanism. BIOPHYS J. 81, 917-936 (2001).
- Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A., Martinac, B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 418, 942-948 (2002).
- Betanzos, M., Chiang, C. -. S., Guy, H. R., Sukharev, S. A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. NAT STRUCT MOL BIOL. 9, 704-710 (2002).
- Corry, B., Jayatilaka, D. Simulation of structure, orientation, and energy transfer between AlexaFluor molecules attached to MscL. BIOPHYS J. 95, 2711-2721 (2008).
- Wang, Y., et al. Single Molecule FRET Reveals Pore Size and Opening Mechanism of MscL. eLife. 3, e01834 (2014).
- Anishkin, A., Akitake, B., Kamaraju, K., Chiang, C., Sukharev, S. Hydration properties of mechanosensitive channel pores define the energetics of gating. J Phys Condens Matter. 22, 454120 (2010).
- Koçer, A., Walko, M., Meijberg, W., Feringa, B. L. A light-actuated nanovalve derived from a channel protein. Science. 309, 755-758 (2005).
- Najem, J., Dunlap, M., Sukharev, S., Leo, D. J. Mechanosensitive Channels Activity in a Droplet Interface Bilayer System. MRS Proceedings. 1621, (2014).
- Fox, R. O., Richards, F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-Å resolution. Nature. 300, 325-330 (1982).
- Iwamoto, M., Oiki, S. Contact Bubble Bilayers with Flush Drainage. Sci Rep. 5, (2015).
- Barriga, H. M., et al. Droplet interface bilayer reconstitution and activity measurement of the mechanosensitive channel of large conductance from Escherichia coli. J R Soc Interface. 11, 20140404 (2014).

