Scaled-Up Preparation of an Intermediate of Upatinib, ACT051-3
Summary
Here, we present a protocol for the scaled-up synthesis of the intermediate tert-butyl (5-toluenesulfonyl-5h-pyrrole [2,3-b] pyrazine-2-yl) carbamate (ACT051-3) of Upatinib.
Abstract
Upatinib, a Janus kinase inhibitor drug, was developed by a biotech company to treat immune diseases. The compound tert-butyl (5-toluenesulfonyl-5h-pyrrole [2,3-b] pyrazine-2-yl) carbamate (ACT051-3) is an important intermediate of Upatinib. To date, the steady industrial production of this intermediate compound (ACT051-3) has not been reported. In this study, we described the specific synthesis method and process of the compound ACT051-3 in terms of laboratory synthesis, pilot scale-up, and industrial production. During the exploration of the process route for ACT051-3, many appropriate adjustments and improvements were made to the reaction conditions, finally leading to the successful development of the optimal industrial production process for ACT051-3. The reaction time was nearly doubled by changing the state of the potassium carbonate involved in the reaction, which greatly improved the reaction efficiency. Additionally, by introducing N,N-diisopropylethylamine (DIPEA) to the reaction, the amount of the expensive catalyst Pd(OAc)2 was reduced 2.5-fold, significantly lowering production costs, confirming the feasibility of this process route and the industrial production of ACT051-3, and satisfying market demand for this important intermediate.
Introduction
Upatinib has become a globally popular Janus kinase 1 (JAK1) inhibitor for treating immune disorders in recent years1,2. This drug has demonstrated significant therapeutic effects on psoriaticarthritis (PsA)3,4, rheumatoid arthritis (RA)5,6,7, and atopic dermatitis (AD)8,9. Additionally, due to its high selectivity10, Upatinib has a wide range of clinical applications. Tert-butyl (5-tosyl-5h-pyrolo [2,3-b] pyrazin-2-yl) carbamate (ACT051-3) is an important intermediate of Upatinib. Its main structural components are the pyrrole ring and the pyrazine ring, which can be used in the preparation of new nitrogen-containing tricyclic kinase inhibitors for the treatment of immune and tumor diseases11.
Pilot scale-up is a medium-sized scale-up (50x-100x) of the process route and conditions determined by the laboratory pilot study, followed by process testing, industrial investigation, and optimization to determine the best industrial production and operating conditions12.
At present, the lab synthesis routes for this intermediate compound (ACT051-3) have been reported, but they have only been performed on a small scale due to problems of low yield, complex reactions, and high equipment requirements, which still have a lot of room to be optimized11,13,14,15. However, no process route has been reported for pilot scale-up and industrial production of the intermediate compound ACT051-3 at present.
Therefore, in this study, we investigated the pilot scale-up and production route of the compound ACT051-3, with reference to the better reported laboratory synthetic routes. Compared with the original laboratory synthesis route, many appropriate adjustments and improvements were made to the reaction conditions, and other factors that may affect the reaction results were investigated. Finally, the most suitable process parameters for the optimal route were identified, and we obtained a process route that is simple to operate, low cost, and environmentally friendly, which is suitable for the pilot scale-up and production of ACT051-3.
Protocol
1. Pilot scale synthesis of compounds ACT051-2 and ACT051-3
- Synthesis of 2-bromo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine (ACT051-2)
- In a round-bottom flask, dissolve 50.0 g of the compound 2-bromo-5H-pyrrolo[2,3-b]pyrazine (ACT051-1; 0.25 M) in 15 mL of N,N-dimethylformamide (DMF; 3 V).
- Add 65.3 g of diisopropylethylamine (DIPEA; 0.51 M) to the reaction solution under nitrogen protection (provide a gas and moisture barrier) and cool the temperature to 0-5 °C through a cool water bath. For nitrogen protection, pump the pressure of the reactor to -0.75–0.8 MPa, then pass N2 to balance the pressure at 0.1 MPa.
- Add 60.20 g of TsCl dissolved in 12 mL of DMF (0.32 M). Raise the temperature to 20-30 °C through the warm water bath and stir for about 1 h. Add cool water (600.0 mL, 0-10 °C) to the mixture and stir for another 1 h.
- Filter the product under a vacuum using a glass funnel with a sanding board padded with filter paper. Wash with water (200.0 mL) several times and dry using an electric thermostatic drying oven to obtain a pale-yellow solid (ACT051-2) with a yield of 78%.
- Synthesis of tert-butyl (5-toluenesulfonyl-5H-pyrrole [2,3-b] pyrazine-2-yl) carbamate (ACT051-3)
- Dissolve 176.11 g of ACT051-2 in 366.31 g of 1,4-dioxane in a three-port round-bottom flask.
- Add 65.75 g of tert-butyl carbamate, 138.21 g of granular potassium carbonate (2.0 eq), 11.57 g of xantphos (0.04 eq), and 2.25 g of Pd(OAc)2 (1.28% wt) to the solution.
- Heat the mixture to 105 °C and stir for 7 h under a nitrogen atmosphere. Allow the mixture to cool to room temperature and filter the product with a Buchner funnel (the filter paper aperture is 80-120 µm).
- Wash the filter residue with ethyl acetate (200 mL). Use a circulating water vacuum pump to concentrate the product under reduced pressure at 50-60 °C with a pressure value of -0.095 MPa. Keep the pump working to maintain the pressure and obtain a dark brown oil.
- Purify the crude product with column chromatography, eluting with petroleum ether and ethyl acetate (V/V, 10/1) to obtain the target compound as a white solid with a yield of 93.5%.
2. Pilot scale-up synthesis of compounds ACT051-2 and ACT051-3
- Pilot scale-up synthesis of 2-bromo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine (ACT051-2)
- Add 1.0 kg of ACT051-1 (5.05 M) and 1.305 kg of DIPEA (10.1 M) to a three-port round-bottom flask. Add 3 L of DMF (3 V) to the flask and dissolve the solid. Heat the reaction mixture to 35 °C.
- Add 1.203 kg of TsCl (6.31 M) to the reaction solution and stir for 1 h. Stir the mixture until the completion of the reaction is confirmed by thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). In brief, take a small amount of reaction product and subject this to TLC monitoring. When the TLC shows that there is almost no raw material left, send the sample to the HPLC central control, and detect the reaction to raw material or the product ratio to be 0.1/99.9.
- Pour in 8.4 L of cool water and stir for another 0.5 h. Filter all liquids with a Buchner funnel (the filter paper aperture is 80-120 µm) and rinse the crude product with 600 mL of water.
- Dry the resulting product at 70 °C overnight using an electric thermostatic drying oven and obtain a product with a yield of 94.9%.
- Pilot scale-up synthesis of tert-butyl (5-toluenesulfonyl-5H-pyrrole [2,3-b] pyrazine-2-yl) carbamate (ACT051-3)
- Add 3.31 L of tert-amyl alcohol and 4.97 L of toluene (V/V, 2/3) to the reaction kettle.
- To the solution, add 1.66 kg of ACT051-2, 0.83 kg of tert-butyl carbamate, 1.301 kg of powdered potassium carbonate, 0.11 kg of xantphos, and 0.31 kg of DIPEA.
- Evacuate nitrogen three times, as in step 1.1.2, repeat this 3x, and add 10 g of Pd(OAc)2 (0.60% wt) to the reaction solution under nitrogen protection.
- Heat the reaction mixture to 90 °C and stir for 4 h. Cool the mixture to 40 °C or below.
- Filter the reaction solution with a Buchner funnel (the filter paper aperture is 80-120 µm) using diatomite as a filter aid and washing the filter cake with toluene.
- Collect and concentrate the filtrate. Use a circulating water vacuum pump to concentrate the filtrate under reduced pressure at 50-60 °C with a pressure value of -0.095 MPa. Keep the pump working to maintain the pressure.
- Add 300 mL of heptane and stir for 20 min. Filter the reaction solution again with a Buchner funnel (the filter paper aperture is 80-120 µm) and rinse the crude product with heptane (50 mL, three times). Dry and obtain the product with a yield of 96.3%.
3. Industrial production of compounds ACT051-2 and ACT051-3
- Industrial production of 2-bromo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine (ACT051-2)
- Confirm that the reactor is clean and free of water, and make sure the mixing device makes no noise when turning on and the bottom discharge valve of the reactor has been closed.
- Add 355.50 kg of DMF (3 V) into the 2,000 L enamel reactor and start to stir. Add 125.04 kg of ACT051-1 (1.0 eq) and 164.8 kg of DIPEA (2.0 eq) to the solution.
- Drop the temperature to 20-25 °C under nitrogen protection. Add 150.22 kg of TsCl (1.25 eq) at 25-35 °C in 10 batches within 3 h.
- Allow the mixture to remain at 25-35 °C and stir for 2 h. Prepare 750.20 kg of cool water (3-4 °C) in another 2,000 L reactor.
- Monitor the reaction by HPLC and confirm completion of the reaction when the remaining raw material is 0.5%.
- Add the cool water ( 3-4 °C) to the reaction mixture and stir at 15-30 °C for 1.5 h. Collect the filtrate and rinse with water (250-500 kg, 2-4 V) until it becomes neutral (tested using pH paper).
- Dry the product at 60-65 °C for 30.5 h to obtain a light brown solid with a yield of 95.5%.
- Industrial production of tert-butyl (5-toluenesulfonyl-5H-pyrrole [2,3-b] pyrazine-2-yl) carbamate (ACT051-3)
- Confirm the reactor is clean and free of water, and make sure the mixing device is normal and the bottom discharge valve of the reactor has been closed.
- Add 340.10 kg of tert-amyl alcohol (2 V) and 552.50 kg of toluene (3 V) into a 2,000 L enamel reactor and start to stir.
- Add 212.40 kg of ACT051-2 (1.0 eq), 106.00 kg of tert-butyl carbamate (1.5 eq), 166.70 kg of powdered potassium carbonate (2.0 eq), 14.10 kg of xantphos (0.04 eq), 39.40 kg of DIPEA (0.5 eq), and 1.06 kg of Pd(OAc)2 (0.5% wt) to the solution.
- Replace the nitrogen four times, allow the mixture to heat to 85-95 °C under nitrogen protection, and stir for 3 h. Monitor the reaction by HPLC and confirm completion of the reaction when the remaining raw material is 0.44%.
- Cool the reaction temperature to 20-30 °C. Collect the solution in batches and place it in a 125 L plastic bucket for 30 min.
- Filter the reaction solution with a chemical suction filter barrel (the filter bag aperture is 10-15 µm) using diatomite as a filter aid and washing the filter cake with toluene (185.20-370.40 kg, 1-2 V).
- Collect the filtrate in an enamel vessel and use a circulating water vacuum pump under reduced pressure at 55-65 °C for 10 h with a pressure value of -0.095 MPa. Keep the pump working to maintain the pressure and obtain the product compound as a viscous liquid.
- Evaporate the product twice by pumping toluene and continuing the reaction. Concentrate the final product for 4 h. Purify the resulting product with column chromatography, eluting with heptane and ethyl acetate (V/V, 10/1-3/1), to obtain a solid with a yield of 98.5%.
Representative Results
This study provides the scaled-up synthesis process for the important intermediate ACT051-3 of Upatinib (Figure 1 and Figure 2). The protocol section (steps 1-3) specifically shows the gram-grade synthesis, pilot-scale kilogram-grade synthesis, and scale-up production step of the compound ACT051-2 and the intermediate ACT051-3.
In the course of exploring the optimal route for the compound ACT051-2, as shown in Table 1, it was found that solid TsCl was more involved in the reaction than liquid TsCl (dissolved in DMF, step 3.1 of the protocol) and significantly reduced the amount of DMF by nearly threefold. In addition, the product yield was increased from 97.49% to 98.44% by increasing the temperature of the mixed solution when TsCl was added from 0-5 °C to 23-35 °C (shown in Table 2). Further, experiments on the consumption of post-treatment water were conducted. As shown in Table 3, after a 2.5-fold reduction in water consumption (from 15 mL/g ACT051-2 to 6 mL/g ACT051-2), the reaction yield decreased by 2.5%, but the waste solution generation was reduced, and the reaction efficiency was significantly improved.
A series of experimental conditions were developed to obtain the optimized process route for the intermediate ACT051-3. As shown in Table 4, by introducing DIPEA into the reaction and replacing the reaction solvent with tert-amyl alcohol/toluene (V/V, 2/3), the amount of Pd(OAc)2 was reduced 2.5-fold (from 1.28% wt to 0.5% wt), which significantly reduced the production cost and further improved the feasibility of scaling up the production. Additionally, by changing the state of K2CO3 involved in the reaction, the reaction time was reduced from 7 h to 3.5 h, which greatly improved the reaction efficiency (as shown in Table 5). Additionally, by switching from tert-amyl alcohol/1,4-dioxane (V/V, 1/4) to tert-amyl alcohol/toluene (V/V, 2/3), the reaction time was shortened to 3 h, the product peak area increased from 84.22% to 88.52%, and the time it took for the product to concentrate was significantly shortened, all of which improved the reaction's efficiency (see Table 6).
Both compounds ACT051-2 and ACT051-3 were chemically characterized by proton nuclear magnetic resonance (1H NMR), HPLC, and high-resolution mass spectrometry. The analysis methods (HPLC, 1H NMR, and electrospray ionization [ESI] spectroscopy) of ACT051-2 and ACT051-3 can be found in the supporting work (Supplementary Table 1, Supplementary Figure 1, Supplementary Figure 2, Supplementary Figure 3, Supplementary Figure 4, Supplementary Figure 5, and Supplementary Figure 6). The characterization data for ACT051-2 and ACT051-3 are reported below:
2-bromo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine (ACT051-2):
1H NMR (500 MHz, DMSO-d6)δ8.59 (s,1H), 8.37 (d, J = 4.1 Hz, 1H), 8.00 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.02 (d, J = 4.0 Hz, 1H), 3.29 (d, J = 11.9 Hz, 3H). ESI: m/z calculated for C13H10BrN3O2S [M] + 352.21, found to be 352.00.
Tert-butyl (5-toluenesulfonyl-5H-pyrrole [2,3-b] pyrazine-2-yl) carbamate (ACT051-3):
1H NMR (500 MHz, CDCl3) δ8.98 (s, 1H), 7.95 (d, J = 8.4 Hz, 2H), 7.84 (d, J = 4.1 Hz, 1H), 7.21 (s, 1H), 7.19-7.17 (m, 1H), 6.53 (d, J = 4.1 Hz, 1H), 2.30 (s, 3H), 1.45 (s, 9H). ESI: m/z calculated for C18H20N4O4S [M+H] + 389.12, found to be389.15.
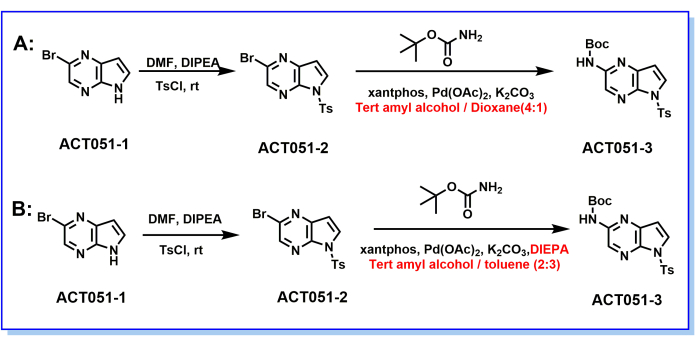
Figure 1: Synthesis route of the intermediate ACT051-3. (A) The reaction route and conditions of ACT051-3 before optimization: i) DMF, DIPEA, TsCl; ii) xantphos, Pd(OAc)2, K2CO3, tert-amyl alcohol/1,4-dioxane (V/V, 1/4); (B) The reaction route and conditions of ACT051-3 after optimization: i) DMF, DIPEA, TsCl; ii) xantphos, Pd(OAc)2, K2CO3, DIPEA, tert-amyl alcohol/toluene (V/V, 2/3). Please click here to view a larger version of this figure.
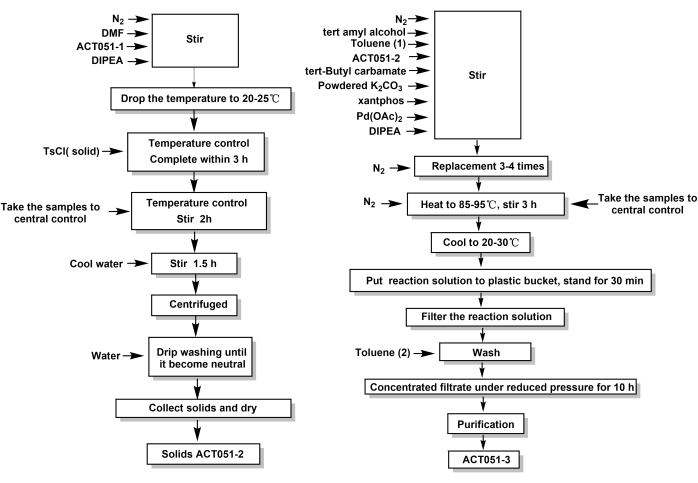
Figure 2: The process flow diagram of compounds ACT051-2 and ACT051-3 in scale-up production. (A) Process flow diagram of ACT051-2 in scale-up production. (B) Process flow diagram of ACT051-3 in scale-up production. Please click here to view a larger version of this figure.
| Number | State of TsCl | V(DMF) (Laboratory test) | V(DMF) (Pilot scale up) |
| 1 | dissolve in DMF | 8.5 V | 54 V |
| 2 | solid | 3.0 V | 18 V |
Table 1: Effect of different forms of TsCl on the synthetic compound ACT051-2. The different states of TsCl include liquid TsCl (dissolved in DMF) and solid TsCl. Experimental results show that solid TsCl is more conducive to industrial production.
| Number | Temperature (°C) | Condition of mix | Whether has a clarification process | Yield | Purity |
| 1 | 0-5 | viscous | No | 97.49% | 96.85% |
| 2 | 25-35 | good mixing | Yes | 98.44% | 96.99% |
Table 2: Effect of adding TsCl at different temperatures on the synthesis of ACT051-2. Adding TsCl to the reaction at 0-5 °C or 23-35 °C.
| Number | Water consumption | Yield | Purity |
| 1 | 15 mL / g ACT051-1 | 97.49% | 96.85% |
| 2 | 6 mL / g ACT051-1 | 94.90% | 97.69% |
| 3 | 9 mL / g ACT051-1 | 95.07% | 96.71% |
Table 3: Effect of different post-treatment water consumption on the synthesis of ACT051-2. Try the different post-treatment water consumptions, including 15 mL/g ACT051-1, 9 mL/g ACT051-1, and 6 mL/g ACT051-1. The optimal conditions were achieved at a post-treatment water volume of 6 mL/g ACT051-2.
| Number | Equivalent of DIEPA | Equivalent of K2CO3 | Equivalent of Pd(OAc)2 |
| 1 | 0.0 eq | 3.0 eq | 1.28% wt |
| 2 | 2.0 eq | 2.0 eq | 0.60% wt |
| 3 | 1.0 eq | 2.0 eq | 0.60% wt |
| 4 | 0.5 eq | 2.0 eq | 0.60% wt |
Table 4: Effect of adding DIPEA to the reaction for the synthesis of ACT051-3. Exploring the effect of DIPEA addition or not on the reaction. The results showed that the introduction of DIPEA reduced the amount of Pd(OAc)2 by a factor of 2.5 (from 1.28% wt to 0.5% wt).
| Number | State of K2CO3 | Equivalent | Reaction time (h) |
| 1 | solid | 2.0 eq | 7 |
| 2 | powdered | 2.0 eq | 3.5 |
Table 5: Effect of different states of K2CO3 on the reaction of the synthetic compound ACT051-3. Select potassium carbonate in granular or powder form to participate in the reaction.
| Number | Dosage of Pd(OAc)2 | Reaction solvent | V/V | Reaction time / h | Product peak area/ % |
| 1 | 0.60% wt | tert amyl alcohol / 1,4-dioxane | 1-4 | 3.5 | 84.22 |
| 2 | 0.60% wt | tert amyl alcohol / 1,4-dioxane | 2-3 | 3.5 | 83.34 |
| 3 | 0.60% wt | tert amyl alcohol / Toluene | 2-3 | 3 | 88.52 |
| 4 | 0.50% wt | tert amyl alcohol / Toluene | 2-3 | 2.25 | 87.11 |
Table 6: Effect of different reaction solvents on the reaction of the synthetic compound ACT051-3. Tert-amyl alcohol/1,4-dioxane (V/V, 1/4) and tert-amyl alcohol/toluene (V/V, 2/3) are selected as reaction resolvents.
Supplementary Table 1: Analytical method of compounds ACT051-2 and ACT051-3. Specific chromatographic conditions for the analysis of compounds ACT051-2 and ACT051-3, including the instrument, method name, liquid phase column, mobile phase, column temperature, current speed, and wavelength. Please click here to download this File.
Supplementary Figure 1: The high-performance liquid chromatograms of ACT051-2. The results for the data were detected by HPLC. Please click here to download this File.
Supplementary Figure 2: The high-performance liquid chromatograms of ACT051-3. The results for the data were detected by HPLC. Please click here to download this File.
Supplementary Figure 3: MS spectrum of ACT051-2. The results for the data were detected by ESI spectroscopy. Please click here to download this File.
Supplementary Figure 4: MS spectrum of ACT051-3. The results for the data were detected by ESI spectroscopy. Please click here to download this File.
Supplementary Figure 5: 1H NMR spectrum of ACT051-2. The results for the data were analyzed using MestReNova. Please click here to download this File.
Supplementary Figure 6: 1H NMR spectrum of ACT051-3. The results for the data were analyzed using MestReNova. Please click here to download this File.
Discussion
The reaction conditions of synthesis, including reaction temperature, time, the selection of reaction reagents, and the ratio of materials, affect the feasibility of the reaction, yields, purity, and production cost, especially for scale-up production.
In the laboratory synthesis of ACT051-2, TsCl in liquid form (dissolved in DMF; step 1.1.3) can be used to in the reaction; however, it is not suitable for pilot scale-up synthesis or industrial production, as using liquid TsCl for this reaction increases the amount of solvent in the reaction system, resulting in more waste fluid. As a result, we chose solid TsCl for the reaction in the pilot scale-up and industrial production (steps 2.1.2 and 3.1.3 of the protocol, respectively) and obtained good experimental results, keeping in mind the concept of environmental protection and green chemistry (Table 1).
In addition, it was found in the pilot scale-up experiments that adding a large amount of TsCl at a low temperature makes the reaction system too viscous to be stirred, resulting in the risk of encapsulation of the raw material. To address this risk, the temperature was increased from 0-5 °C to 25-35 °C (steps 2.1.1 or 3.1.4 of the protocol), sufficient stirring was obtained for the solid TsCl added, the reaction solution system had good fluidity, and the reaction proceeded smoothly (Table 2).
Moreover, a large amount of water (15 mL/g ACT051-1) was required for the post-treatment experiments of ACT051-2, which was not suitable for the scaling up production experiments and generated more waste solution. Therefore, from the perspective of environmental protection, the optimal amount of water used for post-treatment was immediately investigated in the exploration of the production process conditions. As shown in Table 3, when the amount of post-treatment water was reduced to 6 mL/g ACT051-1, the yield of the experiment showed a little impact, but could greatly improve the efficiency and reduce the generation of waste liquid.
In exploring the industrial production process route of the intermediate ACT051-3, we conducted a large number of process optimization experiments and solved many scaling problems. In the synthesis of ACT051-3, ACT051-2 underwent a Buchwald-Hartwig coupling reaction with tert-butyl carbamate catalyzed by Pd(OAc)2 and xanthphos to give the compound ACT051-3. Pd(0) is an active species of palladium commonly used in coupling responses like Suzuki and Buchwald16,17,18,19. However, Pd(II) is commonly used to catalyze the reaction. As a result, we used an amine compound (DIPEA) to reduce Pd(II) to Pd(0), leaving the catalytic system of the entire reaction in a Pd(0) and Pd(II) catalytic cycle. With the addition of DIPEA, the amount of the expensive catalyst Pd(OAc)2 was greatly reduced from 1.28% to 0.5% (Table 4), which greatly reduced the cost of scale-up production and further strengthened the rationality and feasibility of the method.
The long reaction time brings about a large number of side reactions and generates impurities, which is not conducive to scaling up production. Through extensive laboratory studies, it was found that changing the state of potassium carbonate from granular to powder (steps 1.2.2 or 2.2.2 of the protocol) resulted in an appropriate reduction of the reaction time, which is more favorable for industrial production (Table 5). In addition, considering that the 1,4-dioxane used in the small-scale pilot experiment was more difficult to concentrate (step 1.2.1 of the protocol), we considered the combination of toluene and tert-butanol (steps 2.2.1 or 3.2.2 of the protocol; V/V, 2/3), which are better concentrated solvents, as reaction solvents for the industrial production. The results showed that the reaction obtained a great promotion rate, reduced the reaction time, and improved the efficiency of the reaction (see Table 6).
In conclusion, after repeated exploration of the experimental conditions, the optimal process scale-up conditions for compounds ACT051-2 and ACT051-3 were finally obtained, and the process flow diagram of this scale-up production is shown in Figure 2. The results of the scaled-up production showed that the whole process was stable, and the product yield was normal (steps 3.1.7 or 3.2.8 of the protocol).
The industrial production route obtained in this study is a new route available, and the feasibility of this process route in future industrial production was also confirmed. In addition, the results obtained in this study provide some technical research basis for future research of the industrial production route of compounds ACT051-2 and ACT051-3.
However, there is still room for optimization and improvement of the process route, such as the amount of TsCl (1.25 eq), which is excessive in the synthesis of ACT051-2 and can be further reduced. In addition, in the synthesis of ACT051-3, only toluene can be used as the reaction solvent to facilitate post-processing, and the amount of catalyst Pd(OAc)2 may continue to decrease. The above technical issues can be further studied and explored in future work to better scale-up the production of the synthetic compound ACT051-3.
Disclosures
The authors have nothing to disclose.
Acknowledgements
There are no acknowledgments to mention here.
Materials
| 2-bromo-5H-pyrrolo[2,3-b]pyrazine | Nanjing Cook Biotechnology Co., Ltd. | 19120110 | |
| 1,4-dioxane | Liaoning cook Biotechnology Co., Ltd | General Reagent | |
| 1H NMR | Bruker AVIII 500 | ||
| 37% chloride acid molecular grade | NEON | 02618 NEON | |
| 4-toluenesulfonyl chloride (TsCl) | Nanjing Cook Biotechnology Co., Ltd. | AR A2010137 | |
| Anti-Chicken IgY (H+L), highly cross-adsorbed, CF 488A antibody produced in donkey | Sigma-Aldrich | SAB4600031 | |
| Anti-mouse IgG (H+L), F(ab′)2 | Sigma-Aldrich | SAB4600388 | |
| BD FACSCanto II | BD Biosciences | BF-FACSC2 | |
| BD FACSDiva CS&T research beads (CS&T research beads) | BD Biosciences | 655050 | |
| BD FACSDiva software 7.0 | BD Biosciences | 655677 | |
| Bovine serum albumin | Sigma-Aldrich | A4503 | |
| Centrifuge 5702 R | Eppendorf | Z606936 | |
| Circulating water vacuum pump | Guangzhou Zhiyan Instrument Co., Ltd | SHZ-D( ) ) |
|
| CML latex, 4% w/v | Invitrogen | C37253 | |
| Diatomite | Guangzhou Qishuo Chemical Co., Ltd. | / | |
| Double cone rotary vacuum dryer | Jiangsu Yang-Yang Chemical Equipment Plant Inc | SZE-500T | |
| enamel kettle | Jiangsu Yang-Yang Chemical Equipment Plant Inc | CS-03-002 | 1000L / 2000L |
| heptane | Nanjing Cook Biotechnology Co., Ltd. | General Reagent | |
| HPLC | Guangzhou aoyi Technology Trading Co., Ltd | LC-2030C 3D | |
| Large scale rotary evaporators | Guangzhou Xingshuo Instrument Co.,Ltd. | RE-2002 | |
| Low temperature and constant temperature stirring reaction bath | Guangzhou Yuhua Instrument Co., Ltd | XHDHJF-3005 | |
| Low temperature coolant circulating pump | Guangzhou Jincheng Scientific Instrument Co., Ltd | XHDLSB-5/25 | |
| Megafuge 8R | Thermo Scientific | TS-HM8R | |
| N, N-Diisopropyl ethylamine (DIPEA) | Apicci Pharm | General Reagent | |
| N-dimethylformamide (DMF) | Guangzhou bell Biotechnology Co., Ltd | General Reagent | |
| Octanoid acid | Sigma-Aldrich | O3907 | |
| Pd(OAc)2 | Xi'an Catalyst New Materials Co.,ltd. | 200704 | |
| Phosphate buffered saline | Sigma-Aldrich | 1003335620 | |
| Potassium carbonate (K2CO3) | Guangzhou Zhonghua Trade Co.,Ltd. | General Reagent | |
| Tert amyl alcohol | Nanjing Cook Biotechnology Co., Ltd. | General Reagent | |
| tert-Butyl carbamate | Nanjing Cook Biotechnology Co., Ltd. | General Reagent | |
| Thermo Mixer Heat/Cool | KASVI | K80-120R | |
| toluene | Liaoning cook Biotechnology Co., Ltd | General Reagent | |
| Vacuum drying oven | Guangzhou Yuhua Instrument Co., Ltd | DZF-6090 | |
| Water | / | / | |
| Xantphos | Liaoning cook Biotechnology Co., Ltd | Asp20-44892 |
References
- Kerschbaumer, A., et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a systematic literature research. RMD Open. 6 (3), e001374 (2020).
- Fragoulis, G. E., Brock, J., Basu, N., McInnes, I. B., Siebert, S. The role for JAK inhibitors in the treatment of immune- mediated rheumatic and related conditions. The Journal of Allergy and Clinical Immunology. 148 (4), 941-952 (2021).
- Shaw, T., et al. P220 Long-term safety profile of upadacitinib in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis. Rheumatology. 61, 133 (2022).
- Keeling, S., Maksymowych, W. P. JAK inhibitors, psoriatic arthritis, and axial spondyloarthritis: a critical review of clinical trials. Expert Review of Clinical Immunology. 17 (7), 701-715 (2021).
- Fleischmann, R., et al. Safety and efficacy of elsubrutinib or upadacitinib alone or in combination (ABBV-599) in patients with rheumatoid arthritis and inadequate response or intolerance to biological therapies: a multicentre, double-blind, randomised, controlled, phase 2 trial. The Lancet Rheumatology. 4 (6), e395-e406 (2022).
- Stamatis, P., Bogdanos, D. P., Sakka, L. I. Upadacitinib tartrate in rheumatoid arthritis. Drugs of Today. 56 (11), 723-732 (2020).
- Rubbert-Roth, A., et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. The New England Journal of Medicine. 383 (16), 1511-1521 (2020).
- Asfour, L., Getsos Colla, T., Moussa, A., Sinclair, R. D. Concurrent chronic alopecia areata and severe atopic dermatitis successfully treated with upadacitinib. International Journal of Dermatology. 61 (11), e416-e417 (2022).
- Cantelli, M., et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: A case report. Dermatologic Therapy. 35 (4), e15346 (2022).
- Traves, P. G., et al. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Annals of the Rheumatic Diseases. 80 (7), 865-875 (2021).
- Rozema, M. J., et al. Development of a scalable enantioselective synthesis of JAK inhibitor upadacitinib. Organic Process Research & Development. 26 (3), 949-962 (2022).
- Wynn, J. P., Hanchar, R., Kleff, S., Senyk, D., Tiedje, T. Biobased technology commercialization: the importance of lab to pilot scale-up. Metabolic Engineering for Bioprocess Commercialization. , 101-119 (2016).
- Tang, C., et al. . Influenza virus replication inhibitor and use thereof. , (2020).
- Ren, Q., et al. Inhibitors of influenza virus replication and uses thereof. Center for Biotechnology Information. , (2020).
- Van Epps, S., et al. Design and synthesis of tricyclic cores for kinase inhibition. Bioorganic & Medicinal Chemistry Letters. 23 (3), 693-698 (2013).
- Paul, F., Patt, J., Hartwig, J. F. Palladium-catalyzed formation of carbon-nitrogen bonds. Reaction intermediates and catalyst improvements in the hetero cross-coupling of aryl halides and tin amides. Journal of the American Chemical Society. 116 (13), 5969-5970 (1994).
- Zhou, T., Ji, C. L., Hong, X., Szostak, M. Palladium-catalyzed decarbonylative Suzuki-Miyaura cross-coupling of amides by carbon-nitrogen bond activation. Chemical Science. 10 (42), 9865-9871 (2019).
- Sain, S., Jain, S., Srivastava, M., Vishwakarma, R., Dwivedi, J. Application of palladium-catalyzed cross-coupling reactions in organic synthesis. Current Organic Synthesis. 16 (8), 1105-1142 (2019).
- Takale, B. S., Kong, F. Y., Thakore, R. R. Recent applications of Pd-catalyzed Suzuki-Miyaura and Buchwald-Hartwig couplings in pharmaceutical process chemistry. Organics. 3 (1), 1-21 (2021).

