Expression, Purification, and Liposome Binding of Budding Yeast SNX-BAR Heterodimers
Summary
Here, we present a workflow for the expression, purification and liposome binding of SNX-BAR heterodimers in yeast.
Abstract
SNX-BAR proteins are an evolutionarily conserved class of membrane remodeling proteins that play key roles in sorting and trafficking of protein and lipids during endocytosis, sorting within the endosomal system, and autophagy. Central to SNX-BAR protein function is the ability to form homodimers or heterodimers that bind membranes using highly conserved phox-homology (PX) and BAR (Bin/Amphiphysin/Rvs) domains. In addition, oligomerization of SNX-BAR dimers on membranes can elicit the formation of membrane tubules and vesicles and this activity is thought to reflect their functions as coat proteins for endosome-derived transport carriers. Researchers have long utilized in vitro binding studies using recombinant SNX-BAR proteins on synthetic liposomes or giant unilamellar vesicles (GUVs) to reveal the precise makeup of lipids needed to drive membrane remodeling, thus revealing their mechanism of action. However, due to technical challenges with dual expression systems, toxicity of SNX-BAR protein expression in bacteria, and poor solubility of individual SNX-BAR proteins, most studies to date have examined SNX-BAR homodimers, including non-physiological dimers that form during expression in bacteria. Recently, we have optimized a protocol to overcome the major shortcomings of a typical bacterial expression system. Using this workflow, we demonstrate how to successfully express and purify large amounts of SNX-BAR heterodimers and how to reconstitute them on synthetic liposomes for binding and tubulation assays.
Introduction
Membrane-bound organelles such as the plasma membrane, the endoplasmic reticulum, the Golgi apparatus, lysosome (yeast vacuole), and endosome comprise the endomembrane system of the eukaryotic cell. Most organelles have the ability to communicate and exchange material with other organelles through vesicle transport carriers. How the cell coordinates the packaging and formation of vesicle transport carriers within the endomembrane system is not well understood. However, the proteins and lipids that constitute much of the endomembrane system are known to originate from internalizing endocytic vesicles from the plasma membrane (PM). The endosome is the primary acceptor organelle for these vesicles and is comprised of multiple interconnected sets of tubular organelles. The principal function of the endosome is to facilitate nutrient acquisition, regulate protein and lipid turnover, protect from pathogen infection, and to serve as the primary replenishing source of lipids for the plasma membrane. As the endosome receives the bulk of cargo proteins and lipids from the plasma membrane, it acts as a sorting compartment by isolating cargos into tubular endosomal transport carriers (ETCs). Any proteins not sequestered into ETCs are left to be degraded via the endo-lysosomal system. The dysregulation of cargo sorting into ETCs can lead to the loss of nutrient uptake, protein turnover or lipid homeostasis, resulting in numerous metabolic, developmental, and neurological disorders1,2. However, despite ETCs central role at the endosome, the underlying mechanism of how the endosome can selectively coordinate the packaging of a multitude of heterogeneous cargos into tubular carriers is not known.
The sorting nexin (SNX) family is an evolutionarily conserved class of proteins that have been found to be critical for many vesicle transport reactions in the cell3,4,5. Sorting nexins are recruited to the endosome membrane and aid in cargo capture via their characteristic phox homology (PX) domain, which binds phosphatidylinositol-3-monophosphate (PtdIns(3)P), a lipid enriched on the endosome membrane. Mammals encode thirty-three SNX proteins, which can be further divided into multiple subfamilies, according to the presence of other domains1. Most notably, the SNX-BAR subfamily is the largest subfamily consisting of twelve in human, while in budding yeast, Saccharomyces cerevisiae, the subfamily is reduced to just seven SNX-BARs. SNX-BAR proteins have both a PX domain and a Bin-Amphiphysin-Rvs (BAR) domain that triggers lipid reservoirs to bind positive curvature membranes. Consequently, the SNX-BAR family has a natural affinity for the endosome and can mediate ETC formation via their membrane remodeling abilities. In vitro, the remodeling properties of SNX-BARs can be induced by the addition of purified SNX-BARs to synthetic liposomes and the subsequent formation of narrow, coated tubules can be visualized by electron microscopy. Using these methods, researchers have determined that both oligomerization concentration and constriction strength appear to vary amongst the SNX-BAR family suggesting they could aid in both the formation and scission of ETCs.
The SNX-BARs can be further classified by their exclusive dimerization properties. In vitro binding assays and structural studies have demonstrated that SNX-BAR proteins can only form specific homodimers or heterodimers. Therefore, in principle, each potential SNX-BAR dimer-oligomer could provide a tubule coat for a cargo-specific trafficking pathway and likewise, the restricted oligomerization of the other SNX-BAR protomers, can also define distinct export pathways. However, due to the large number of SNX-BARs and diversity within the SNX family, a one sorting nexin-one cargo hypothesis is highly unlikely. Instead a coordinated effort using a multitude of factors such as SNX-BARs, cargo, lipid specificity and other dependencies is more probable. Likewise, recent studies of the yeast SNX4 family revealed evidence for additional lipid specificity, beyond PtdIns(3)P, to potentiate endosome transport carriers6. In this study, SNX-BAR homodimer Mvp1-Mvp1 was purified from bacteria and native heterodimers Snx4-Atg20 and Vps5-Vps17 were expressed and purified in high yield from yeast, while only Snx4-Atg20 was found to preferentially bind phosphatidylserine (PS) and form narrow tube-like structures in liposome binding studies6. While others in the field have revealed important properties of the SNX-BARs using recombinantly purified SNX-BAR homodimers from bacteria, toxicity associated with expressing SNX-BAR heterodimers in similar systems have hindered their native characterization7,8,9,10. Therefore, without a reliable system to obtain pure recombinantly expressed native heterodimers, researchers must forgo these lines of investigation. In Figure 1, we present a four-part workflow to 1) construct a yeast strain overexpressing SNX-BAR heterodimers for tandem affinity purification, 2) express and purify native SNX-BAR heterodimers, 3) prepare unilamellar synthetic liposomes, and 4) set up a liposome tubulation or sedimentations assay, providing a vital tool for researchers to investigate the growing catalogue of sorting nexins found in nature.
Protocol
1. Yeast Strain Construction
- Begin with TVY614 (pep4Δ::LEU2 prb1Δ::hisG prc1Δ::HIS3)11 as the parent strain. This strain is deficient for vacuolar proteases, which contribute to the majority of protein degradation after cell lysis, and therefore allows for a cleaner and more efficient purification.
- Design primers12 and integrate tandem affinity purification (TAP) tag at the C-terminus of Atg20 (SNX-BAR ORF 1) using homologous recombination. Use polymerase chain reaction (PCR) to confirm integrations (Figure 2).
- Perform a western blot of cell lysate against the TAP tag to confirm proper integration13.
NOTE: We recommend harvesting 3 OD (1 OD ≈ 1 x 107 cells) of cells for SDS-PAGE and western blot verification. Note that integration of the TAP tag should occur prior to replacing the endogenous promoters with the GAL1 promoter to allow for easier TAP tag verification via western blot. - Replace endogenous Snx4 (SNX-BAR ORF1) and Atg20 (SNX-BAR ORF 2) promoters with untagged GAL1 promoter using sequential homologous recombination and transformation steps of each individual ORF14.
- Use flanking primers outside of the integration sites to PCR confirm successful integrations (Figure 2). This will result in a null phenotype of the targeted SNX-BARs in the absence of galactose in the growth medium.
2. Yeast Induction and SNX-BAR Dimer Purification
NOTE: Yeast cells can be propagated on standard YPD (yeast extract, peptone, and 2% glucose) agar plates as the modifications are chromosomally integrated.
- Inoculate a large swab of cells into 50 mL of standard YP (yeast extract and peptone) medium with 2% raffinose and 0.1% glucose as carbon source in a flask at least 4x the volume of the culture and grow overnight in 30 °C shaker to allow proper aeration. Expect that growth in this medium will be slower compared to standard YPD.
- Next morning, use the 50 mL preculture to inoculate into 1 L of standard YP medium with 2% raffinose and 0.1% glucose and grow for 4-5 h in 30 °C shaker.
NOTE: The volume of preculture used to inoculate 1 L culture may be adjusted depending on the OD600 of the preculture. The OD600 of the 1 L culture after inoculation should be around 0.2 to allow for at least two doublings during the 4-5 h growth.- Use a baffled Fernbach flask for growth to allow proper aeration, a 2.8 L volume flask is sufficient. Less aeration may result in slower growth and smaller cell pellet upon harvesting.
- Check OD600 to make sure culture is in the log phase (0.5-1) after 4-5 h of growth. Depending on the growth of the strain, make adjustments to the growth time to allow for at least two doublings. Add to 2% galactose and grow overnight in 30 °C shaker.
NOTE: The OD600 after the overnight growth may vary but culture should be saturated. Note that cells do not need to be removed from 0.1% glucose before addition of 2% galactose for this growth protocol. We recommend harvesting 3 OD of uninduced and induced cells at this step for SDS-PAGE and western blot for verification (Figure 3A,B, Lane 1-2). - Harvest cells by centrifugation at 4500 x g for 15 min. A swinging bucket rotor that accommodates the 1 L volume culture may be used here.
- Transfer the yeast pellet into a 50 mL conical tube; a second centrifugation step may be performed as needed. The cell pellet will generally be around 10-15 mL in volume as measured by graduation markings and may be used immediately or stored at -80 °C.
- Resuspend pellet in 15 mL purification buffer (50 mM Tris pH 7.4, 300 mM NaCl, 1.5 mM MgCl2, 1 mM Dithiothreitol (DTT), protease inhibitor cocktail) to make final volume around 30 mL.
- Chill a homogenizer 4 °C before use and equilibrate it with purification buffer. Lyse cells using a mechanical cell disruptor or homogenizer. Load sample into the homogenizer and lyse at 20,000-25,000 psi for 2-3 rounds; note that as cells become lysed, more input pressure is required to maintain 20,000-25,000 psi. Collect the cell lysate in a 50 mL conical tube on ice.
NOTE: If additional samples need to be lysed, the homogenizer should be thoroughly cleaned and equilibrated before loading the next sample. Keep all cell lysates on ice. - Immediately clear the cell lysate at 35,000 x g for 1 h at 4 °C. Carefully transfer the supernatant into new tube. Note that the lipids from cell lysis will float to the top during centrifugation and will not affect purification.
NOTE: We recommend saving 0.5-1% of the lysate and pellet for SDS-PAGE samples. Typically, no major differences are observed and an additional western blot may be done to confirm TAP protein solubility (Figure 4, Lanes 1 and 2, respectively). - Equilibrate 300 µL of IgG sepharose beads with purification buffer. Add to the cleared cell lysate and incubate for 2 h, rotating at 4 °C.
- Collect beads in a 10 mL chromatography column and allow unbound lysate to flow through.
- Wash beads using 10 mL of wash buffer (50 mM Tris pH 7.4, 300 mM NaCl, 1.5 mM MgCl2, 1 mM DTT), adding 1 mL at a time and allowing it to flow through completely.
NOTE: We recommend saving 2% of the bound-IgG beads for SDS-PAGE sample. Typically, we observe four major bands; two SNX-BAR proteins and IgG Heavy and Light Chains (Figure 4, Lane 3). We recommend saving equivalent amounts of 'eluate' and 'IgG beads after TEV' to compare for TEV cleavage efficiency. - Collect beads and transfer them to a microcentrifuge tube. Add to 500 µL of total volume with fresh wash buffer and 2 µL of 10 mg/mL TEV protease and incubate overnight, rotating at 4 °C.
- Next morning, remove the supernatant completely using a 27 G needle and assess protein purity by 10% polyacrylamide SDS-PAGE (Figure 4, Lane 4).
NOTE: We typically obtain 500 µL of 0.5-1 mg/mL of 95% pure heterodimer (Figure 4, Lane 4). Additional purification using calmodulin resin can also be done, however we typically see a significant reduction in yield and recommend stopping here if purity is >90%. TEV does not interfere with liposome binding assays, though TEV can be additionally removed using Ni-NTA agarose beads15. - To concentrate, transfer the sample to a 0.5 mL centrifugal filter with 10 KDa cutoff and centrifuge according to manufacturer's instructions to 50 µL or less. Quantify the concentrated proteins using the Bradford protein assay. Store at 4 °C and use within one week.
3. Liposome Preparation
- Purchase commercially available lipids: phosphatidylserine (PS), PI3P, ergosterol, and phosphatidylcholine (PC). If needed, resuspend in recommended solvent to make stock lipids.
NOTE: Lipids are resuspended in a methanol/chloroform mixture per manufacturer's recommendations. Make sure lipid stocks are clear and warmed to room temperature before using. Resuspended lipids can be stored under argon gas and sealed using wax film (or equivalent) at -20 °C for 6-12 months or until loss of activity is observed. - Calculate the volume required of each lipid stock to create a mixture with the desired lipid composition (see Table 1). Assume a total of 1 mole of lipids in the lipid mixture.
- Perform this step in a chemical fume hood. Clean glass syringes by drawing up a full syringe volume of chloroform and discarding it in a waste container. Repeat two more times. When transferring chloroform, use only glass syringes or pipettes. When drawing up chloroform, pull on the stopper slowly to prevent introduction of gas bubbles into the syringe.
- Use glass syringes to transfer stock lipids as calculated into a clean glass culture tube to make a final lipid mixture of 1% PI3P, 20% ergosterol, 30% PS, PC (Table 1). Depending on the solvents each lipid is resuspended in, the mixture may turn cloudy upon addition of each lipid.
NOTE: To vary concentrations of PS (0-30%), adjust volumes accordingly and compensate with varying PC (Table 1). - Carefully dry down the lipid mixture using nitrogen gas directed at the lipid mixture in a circular motion to dry lipids uniformly. Use low gas flow to keep the lipids at the bottom of the glass tube during the drying process. Wrap the glass culture tube with foil, leaving the opening uncovered, and further dehydrate in vacuum for 1 h.
- Add 400 µL of binding buffer (50 mM Tris pH 7.4, 300 mM NaCl, 1 mM MgCl2) to completely dehydrate lipids to make a final liposome concentration of 2.5 mM.
NOTE: Final liposome concentration can be adjusted by adding more or less binding buffer. For instance, 200 µL binding buffer may be added to produce a liposome concentration of 5 mM if needed (see below).- Resuspend lipids by shaking on medium speed on a vortex at room temperature for 30 min. Buffer should appear cloudy as lipids are resuspended.
- Transfer resuspended liposomes to a microcentrifuge tube. From this point on, plastic pipette tips may be used as lipids are no longer resuspended in chloroform. Note that the liposome solution should look cloudy.
- Freeze-thaw liposomes seven to eight times by submerging the microcentrifuge tube first in liquid nitrogen, then in a 37 °C water bath. The liposome mixture should appear completely frozen and solid by eye before thawing.
- Perform steps involving chloroform in a chemical fume hood. Clean two 1 mL glass syringes by drawing up and discarding full syringe volumes of chloroform, three times each, to remove any residual lipids. Equilibrate each glass syringe with ultrapure water by drawing up two syringe volumes, then equilibrate with binding buffer by drawing up two syringe volumes.
- Assemble the mini-extruder according to manufacturer's recommendations. Equilibrate one 200 nm membrane and two pieces of filter supports (see Table of Materials) by submerging each in binding buffer.
- Sandwich the membrane between the filter supports and place in the mini-extruder. To reduce the dead volume in the assembled mini-extruder and to make sure the assembly is air-tight, pass a volume of binding buffer comparable to the volume of the liposome mixture through mini-extruder using the 1 mL glass syringes.
- Use one of the 1 mL glass syringes and draw up the liposome mixture. Invert the microcentrifuge tube to collect the last of the liposome mixture in the tube cap for drawing up into the glass syringe.
- Extrude liposomes by passing through the 200 nm membrane 19-21 times. Collect extruded liposomes in new a microcentrifuge tube.
NOTE: Extruded liposomes should appear less cloudy than liposomes before extrusion. Liposomes should be used the same day and stored on ice. The last extrusion should place liposomes in the syringe opposite of the one it began in.
4. SNX-BAR Liposome Binding and Tubulation
- Preclear purified protein at 100,000 x g in an ultracentrifuge for 20 min at 4 °C prior to conducting liposome binding and sedimentation experiments. Remove the supernatant and transfer it to a new microcentrifuge tube; do not disturb the pellet if there is one.
- To perform liposome binding and tubulation assays, incubate 4 µM purified Snx4-Atg20 and 2.5 mM liposomes in a total reaction volume of 20 µL, varying the volume of liposomes added.
NOTE: In the same experiment, the same volume of liposomes should be used. - Incubate the reaction at 30 °C for 30 min.
NOTE: We suggest maximizing the amount of 2.5 mM liposomes added to each reaction by using no less than 10 µL liposomes to allow for visualization during sedimentation. If 10 µL of 2.5 mM liposomes is used in a 20 µL reaction, the final concentration of liposomes will be 1.25 mM. If the purified proteins are dilute and more volume is required for 4 µM protein, the lipids may be resuspended in 200 µL during the rehydration step to double the concentration of liposomes (see Step 3.6); however, this will require knowing the protein concentration prior to making the liposomes. - Visualize and quantify liposome tubulation.
- Process liposome binding reactions immediately for electron microscopy analysis. Spot samples onto a carbon-coated copper mesh grid and negative stain using 1% uranyl acetate (Figure 5B)16.
- Analyze samples on a transmission electron microscope (200 kV).
- Use image analysis software to measure and quantify tubule diameter. To accurately quantify tubule diameter of a single tubule, take three diameter measurements along the length of a tubule and average (Figure 5B).
NOTE: Two-way analysis of variance was used to determine statistical significance (Figure 5E). Uranyl acetate is both radioactive and toxic. Proper lab safety certification is required to perform this step.
- Liposome binding and sedimentation.
- Transfer the reaction (20 µL, from step 4.3) to a polycarbonate centrifuge tube and use a compatible rotor to spin at 100,000 x g in an ultracentrifuge for 20 min at 4 °C. Carefully remove the supernatant and transfer to new microcentrifuge tube. Note that the pellet should stay intact.
- Resuspend the pellet in SDS-PAGE 40 µL of sample buffer and transfer to a new microcentrifuge tube. Add 20 µL of sample buffer to the supernatant. Load equivalent amounts of pellet and supernatant in a 10% polyacrylamide SDS-PAGE gel and perform Coomassie staining to visualize SNX-BARs bound to liposomes (Figure 5A).
- To quantify the amount of SNX-BAR complex in the pellet fraction, quantify band intensities using densitometry and quantify the proportion of SNX-BAR proteins in the pellet fraction.
NOTE: Two-way analysis of variance was used to determine statistical significance (Figure 5C).
Representative Results
This protocol describes a method for reproducible and robust production of endogenous yeast SNX-BAR complexes that can be used for downstream membrane remodeling assays (Figure 1). The construction of the yeast strain used for purification takes advantage of the efficiency of homologous recombination in budding yeast, allowing for modifications at the genomic loci of the targeted SNX-BARs (Figure 2). This design has two advantages, (i) as selection is not required to maintain the modifications, standard YP medium can be used, allowing for growth to a higher cell density and thus higher production of protein and (ii) the expression levels of the targeted SNX-BARs will be even, optimizing production of the heterodimer complex. Prior to addition of galactose, the targeted SNX-BARs will exhibit a null phenotype and thus may result in a growth defect or other known defects specific to the targeted SNX-BARs. Furthermore, growth in 2% raffinose and 0.1% glucose is slower than growth in 2% glucose. Therefore, the growth period prior to galactose induction may require optimization for each particular strain. To check for proper induction of SNX-BAR expression, a western blot against the TAP tag is likely required since protein levels of the SNX-BARs may not be detected via Coomassie stain (Figure 3). However, because only one member of the SNX-BAR complex has a tag, expression of the untagged protein(s) cannot be confirmed unless all steps of the purification are completed. After purification of the SNX-BAR heterodimer, the bands of the two SNX-BARs should appear to be in 1:1 stochiometric ratio and there should be little to no contaminating bands (Figure 4, lane 4). If there are additional contaminating bands, and the starting yeast strain is already protease-deficient, more protease inhibitor may be added during cell lysis. Furthermore, a second purification step using calmodulin resin may be performed.
When conducting membrane remodeling assays (Figure 5), the same preparation of liposomes and purified proteins must be used in the same experiment. If multiple purification preparations of protein are required to reach the desired concentration, combine all of the protein purified prior to conducting experiment. Liposomes should be made and used within the same day (Table 1). When conducting liposome sedimentation assays, it is critical that the purified protein is precleared using the same sedimentation conditions of 100,000 x g for 20 min immediately prior to incubating with liposomes as precipitated protein may skew results. Furthermore, the liposome pellet should stay intact after sedimentation.
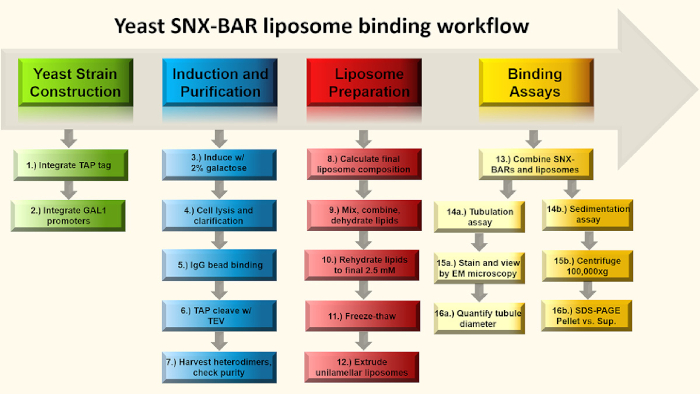
Figure 1: SNX-BAR binding assay flow chart. Briefly, in steps 1-2, we engineer GAL promoters into two SNX-BAR genomic loci, replacing each of the endogenous promoters and engineer a C-terminal TAP tag into one of the two SNX-BAR loci. Next, in steps 3-7, we induce the cells with galactose and purify the SNX-BAR heterodimers to homogeneity. In steps 8-12, we calculate and prepare unilamellar liposomes. Lastly, we can combine the SNX-BAR heterodimers with unilamellar liposomes and perform two assays; Step 14a-16a involve membrane tubulation and 14b-16a involve the sedimentation assay. See text for more details. Please click here to view a larger version of this figure.
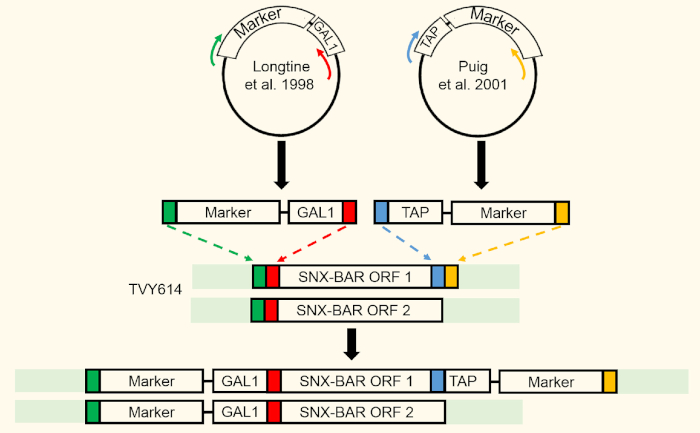
Figure 2: SNX-BAR integration strategy. Two SNX-BAR loci were targeted for GAL expression using homologous recombination. SNX-BAR ORF1 (Atg20) was additionally targeted to express a C-terminal TAP tag. Please click here to view a larger version of this figure.

Figure 3: Galactose induction verification. (A-B) In order to verify GAL induction of Atg20-TAP, we recommend an SDS-PAGE and western blot analysis of cell extracts induced with 2% galactose (A, B, lane 2) and uninduced (A, B, lane 1). (C) Western blot membrane is also stripped and probed with anti-pgk1 for a loading control. Please click here to view a larger version of this figure.
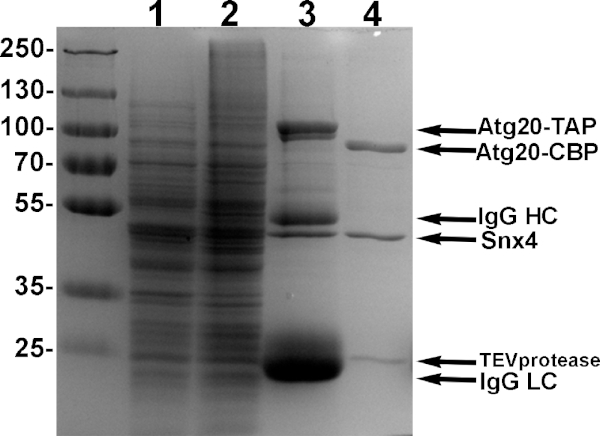
Figure 4: Example purification of Atg20-Snx4 heterodimers. Yeast cells engineered to express Atg20-TAP and Snx4 driven by galactose promoters were induced with 2% galactose, lysed and bound using IgG sepharose, and eluted with TEV protease. Samples from each step of purification is shown in 10% SDS-PAGE. Lane 1: induced supernatant of lysate. Lane 2: induced pellet of lysate. Lane 3: sample of bound proteins to IgG sepharose. Lane 4: TEV eluate of pure Atg20-Snx4 heterodimers from IgG sepharose. Please click here to view a larger version of this figure.
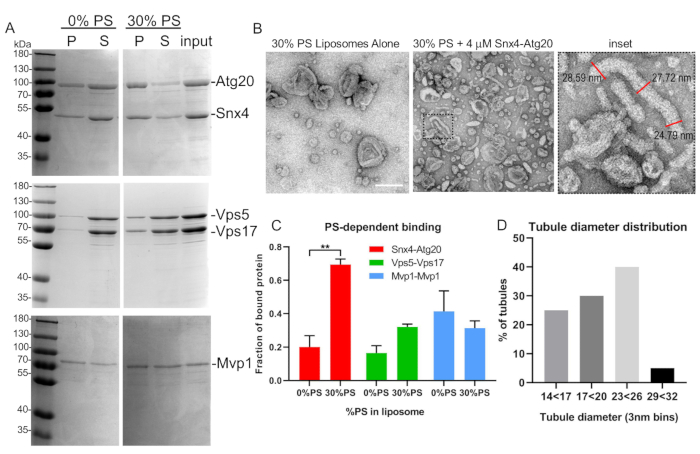
Figure 5: Representative liposome binding and tubulation assay. (A) SNX-BAR heterodimers, Atg20-Snx4 and Vps5-Vps17 from yeast, were expressed, purified, and bound to synthetic liposomes as described in text. Note that Mvp1 forms homodimers and was expressed in bacteria. (B) EM micrographs of Snx4-Atg20 liposome tubulation assay and tubule measurements (inset). (C) SNX-BAR binding to varying liposome compositions was quantified by densitometry. Graph indicates mean and standard error of the mean. **p < 0.002. (D) Tubule diameters were quantified and graphed as described in the text. Scale bar = 200 nm. Error bars represent two-way analysis of variance. Please click here to view a larger version of this figure.
| Typical Liposome Composition | DOPC | DOPS | Ergosterol | PI3P-C16 |
| MW | 786.1 | 810.0 | 396.7 | 957.0 |
| mol fraction | 49% | 30% | 20% | 1% |
| Stock mM | 32.0 | 12.0 | 25.0 | 1.0 |
| Mass (mg) | 385.2 | 243.0 | 79.3 | 9.6 |
| Concentration (mg/mL) | 25.2 | 9.7 | 9.9 | 1.0 |
| Volume to RXN(mL) | 15.3 | 25.0 | 8.0 | 10.0 |
Table 1: Liposome recipe. Synthetic liposomes were prepared using a combination of DOPC, DOPS, ergosterol, and PI3P. We calculate the final concentration to 1 mol of lipid. Our standard composition includes 20% ergosterol, 1% PI3P, DOPS (up to 30%), and varying amounts of DOPC. Table includes a typical formulation for 400 µL of 30% DOPS.
Discussion
Here, we demonstrate an optimized workflow to purify SNX-BAR dimers in yeast and two assays to evaluate their biophysical properties on synthetic liposomes. The main advantage over typical recombinant protein expression in Escherichia coli or other systems is the ability to evenly express SNX-BAR proteins in a native host, thus avoiding the toxicity and insolubility issues found in purifying SNX-BARs in other systems. It is also notable that our system does not require molecular cloning or the harboring of multiple expression vectors17. Our dual SNX-BAR yeast strains are driven by chromosomally engineered galactose promoters, thus ensuring even expression upon induction. An important consideration is that in the absence of galactose, there will be little to no expression of the targeted SNX-BARs, thus resulting in a null phenotype. Furthermore, we find that SNX-BARs tend to tolerate C-terminal tags well, allowing us to add the TAP tag at the C-terminus. However, depending on the protein being tagged, an N-terminal TAP tag may also be used12. Additionally, since SNX-BARs only form 1:1 dimers, only one protein is required to be tagged for purification purposes. However, some SNX-BARs that normally form heterodimers in the cell have been shown to form homodimers under non-physiological concentrations7. Therefore, it is critical that upon purification of the heterodimer, the stoichiometric ratio of the two SNX-BARs should be 1:1, which can be verified by running an aliquot of the purified complex on an SDS-PAGE gel and performing Coomassie staining. Once engineered, these yeast strains can be saved in perpetuity as 15% (v/v) glycerol stock at -80 °C and/or additionally modified to query additional binding partners. Our workflow typically takes 2-3 weeks for strain construction and 3-4 days for expression and purification and 1 day for liposome binding assays. We believe that this workflow can help researchers further understand the lipid specificity of SNX-BAR proteins using native BAR proteins on synthetic liposomes or giant unilamellar vesicles (GUVs) and reveal the precise makeup of lipids needed to drive membrane remodeling, thus revealing their mechanism of action.
Critical steps
During our first attempts at purifying SNX-BAR heterodimers from non-protease deficient cells, we often found reduced yields and degradation products. Therefore, during the initial steps of strain construction, we believe it is critical to begin with a parental yeast strain that is deficient for one or more of the major vacuolar proteinases. In particular, we found yeast strain TVY614, which is depleted for pep4, prb1, and prc1, to be the most optimal. Using the TVY614 strain, we routinely obtain >90% pure Snx4-Atg20 heterodimers (Figure 4 and Figure 5A). However, the necessity for all three proteinases to be ablated may be SNX-BAR combination specific. For example, Vps5-Vps17 heterodimers have been successfully purified in non-protease deficient strains10 and when we included the addition of a PEP4 ablation, we observe modest increases in yield and purity (Figure 5A). Therefore, depending on the user's downstream applications and the need for purity or selectable markers, there may be flexibility when designing expression strains.
The order of gene construction is also important. We recommend C-terminally TAP tagging SNX-BAR ORF 1 first, in order to confirm expression by western blot without the need for galactose induction (Figure 1). During galactose induction, it is critical to pre-condition the cells overnight in 2% raffinose and 0.1% glucose. Failure to pre-condition the cells results in extremely slow growth or cell death. However, it is also critical for the cells to deplete the remaining glucose during overnight growth, otherwise galactose induction can be negatively impacted. It is also recommended to check multiple isolates by western blot to evaluate expression homogeneity of the TAP tagged SNX-BAR proteins. We typically screen 2-3 isolates and pick the most robustly expressing candidate.
Modification, alternative approaches, and future applications
In step 2.8, tandem affinity purification (TAP) typically requires a two-step affinity purification using IgG and calmodulin beads after TEV cleavage12. However, in this protocol, we elute SNX-BAR dimers by TEV protease with very high yield and purity. We find subsequent affinity purification using calmodulin beads produces inconsistent and reduced yields, thus we recommend stopping after TEV cleavage. The TEV eluate containing the SNX-BAR proteins and His(6)-tagged TEV protease can be further purified by Ni-NTA agarose beads. However, we also find this step can reduce overall SNX-BAR protein yield and is unnecessary, since TEV protease does not interfere with liposome binding or tubulation assays. Therefore, if the purified SNX-BARs will be used for any other application, we recommend the user assess the impact of TEV protease in their assays.
Thus far, we have been successful using this protocol and the described modifications to purify Snx4-Atg20 and Vps5-Vps17 heterodimers in yeast and have successfully assessed their lipid specificity on synthetic liposomes. However, we believe that the protocol can be successfully adapted to any of the SNX-BARs in yeast. It is also possible to use the system to produce recombinant SNX-BAR proteins from any other organisms. However, this would require an additional step of strain construction to integrate an exogenous gene locus into the yeast genome. We also believe the system can be expanded to purify multimeric complexes including cargo proteins. Thus, we believe our expression system may extend beyond understanding the lipid specifies of SNX-BARs. Future applications will allow researchers to reconstitute whole cargo capture complexes on liposomes to understand how full assemblies can influence membrane remodeling.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number GM060221 and in part by the National Institute of General Medical Sciences of the National Institutes of Health under award number T32GM007223. R.C. was supported in part by the UNC-Charlotte Faculty Research Grants Program.
Materials
| 0.2 micrometer PC Membranes | Avanti | 610006 | |
| 10 mL Poly-Prep Chromatography column (Bio-Rad) | Bio-Rad | 731-1550 | |
| 27 Gauge needle | BD Biosciences | 301629 | |
| Amicon Ultra Centrifugal Filter with 10K cutoff | Amicon | UFC501024 | |
| Avestin EmulsiFlex-C3 Homogenizer | Avestin | EF-C3 | |
| BCA assay | Pierce | 23225 | |
| Beckman Optima MAX-XP Ultracentrifuge | Beckman Coulter | 393315 | |
| cOmplete Protease Inhibitor Cocktail | Roche | 4693116001 | |
| DOPC | Avanti | 850375 | |
| DOPS | Avanti | 840035 | |
| ergosterol (Sigma) | Sigma | 47130-U | |
| Extruder Set with Block 0.2 microlter/1mL | Avanti | 610000 | |
| FEI Tecnai F20 transmission electron microscope (200 kV) | |||
| Glass culture tubes | VWR | 47729-570 | |
| IgG sepharose beads (GE Healthcare) | GE Healthcare | 17-0969-01 | |
| Microlter glass syringes | Hamilton | 7637-01 | |
| New Brunswick Excella E25 | Eppendorf | M1353-0000 | or equivalent shaking 30 C |
| Ni-NTA Magnetic Agarose Beads | Pierce | 78605 | |
| Optima XE-90 Ultracentrifuge | Beckman Coulter | A94516 | |
| Parafilm M | VWR | 52858-076 | |
| PI3P | Echelon | P-3016 | or Echelon equivalent |
| Polycarbonate bottle assembly | Beckman Coulter | 355622 | |
| TLA-100 Fixed-Angle Rotor | Beckman Coulter | 343840 | |
| Type 45 Ti Rotor | Beckman Coulter | ||
| Vacuum Desiccator, Bottom and Lid with Socket Valve | VWR | 75871-436 | |
| Vacuum Pump Alcatel (Pascal 2005 C1) | A&J Vacuum | PN07050 | |
| Vortex with foam holder | VWR | 10153-838 | |
| VWR KIT MICROTUBE | VWR | 12620-880 | |
References
- Teasdale, R. D., Collins, B. M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. The Biochemical Journal. 441 (1), 39-59 (2012).
- Zhang, H., et al. The Retromer Complex and Sorting Nexins in Neurodegenerative Diseases. Frontiers in Aging Neuroscience. 10, 79 (2018).
- Burd, C., Cullen, P. J. Retromer: a master conductor of endosome sorting. Cold Spring Harbor perspectives in Biology. 6 (2), (2014).
- Chi, R. J., Harrison, M. S., Burd, C. G. Biogenesis of endosome-derived transport carriers. Cellular and Molecular Life Sciences. 72 (18), 3441-3455 (2015).
- Wang, J., et al. Endosomal receptor trafficking: Retromer and beyond. Traffic (Copenhagen, Denmark). 19 (8), 578-590 (2018).
- Ma, M., et al. Lipid trafficking by yeast Snx4 family SNX-BAR proteins promotes autophagy and vacuole membrane fusion. Molecular Biology of the Cell. , (2018).
- van Weering, J. R., et al. Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. The EMBO Journal. 31 (23), 4466-4480 (2012).
- Chi, R. J., et al. Fission of SNX-BAR-coated endosomal retrograde transport carriers is promoted by the dynamin-related protein Vps1. The Journal of Cell Biology. 204 (5), 793-806 (2014).
- Purushothaman, L. K., Arlt, H., Kuhlee, A., Raunser, S., Ungermann, C. Retromer-driven membrane tubulation separates endosomal recycling from Rab7/Ypt7-dependent fusion. Molecular Biology of the Cell. 28 (6), 783-791 (2017).
- Purushothaman, L. K., Ungermann, C. Cargo induces retromer-mediated membrane remodeling on membranes. Molecular Biology of the Cell. 29 (22), 2709-2719 (2018).
- Wurmser, A. E., Emr, S. D. Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. The EMBO journal. 17 (17), 4930-4942 (1998).
- Puig, O., et al. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 24 (3), 218-229 (2001).
- Kushnirov, V. V. Rapid and reliable protein extraction from yeast. Yeast. 16 (9), 857-860 (2000).
- Longtine, M. S., et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 14 (10), 953-961 (1998).
- Tropea, J. E., Cherry, S., Waugh, D. S. Expression and purification of soluble His(6)-tagged TEV protease. Methods in Molecular Biology. 498, 297-307 (2009).
- Zhang, L., et al. Morphology and structure of lipoproteins revealed by an optimized negative-staining protocol of electron microscopy. Journal of Lipid Research. 52 (1), 175-184 (2011).
- Yong, X., et al. Expression and purification of the SNX1/SNX6 complex. Protein Expression and Purification. 151, 93-98 (2018).

