Micromanipulation of Gene Expression in the Adult Zebrafish Brain Using Cerebroventricular Microinjection of Morpholino Oligonucleotides
Summary
In this article, we demonstrate a method for manipulation of gene expression in the ventricular cells of the adult zebrafish telencephalon using antisense morpholino oligonucleotides. We present this method as an efficient and quick protocol that can be used for functional studies in the adult vertebrate brain.
Abstract
Manipulation of gene expression in tissues is required to perform functional studies. In this paper, we demonstrate the cerebroventricular microinjection (CVMI) technique as a means to modulate gene expression in the adult zebrafish brain. By using CVMI, substances can be administered into the cerebroventricular fluid and be thoroughly distributed along the rostrocaudal axis of the brain. We particularly focus on the use of antisense morpholino oligonucleotides, which are potent tools for knocking down gene expression in vivo. In our method, when applied, morpholino molecules are taken up by the cells lining the ventricular surface. These cells include the radial glial cells, which act as neurogenic progenitors. Therefore, knocking down gene expression in the radial glial cells is of utmost importance to analyze the widespread neurogenesis response in zebrafish, and also would provide insight into how vertebrates could sustain adult neurogenesis response. Such an understanding would also help the efforts for clinical applications in human neurodegenerative disorders and central nervous system regeneration. Thus, we present the cerebroventricular microinjection method as a quick and efficient way to alter gene expression and neurogenesis response in the adult zebrafish forebrain. We also provide troubleshooting tips and other useful information on how to carry out the CVMI procedure.
Introduction
Adult neurogenesis is a common trait to vertebrates, however its degree of prevalence and efficiency varies with the phylogeny 1,2,3,4,5,6. For instance, adult mammalian brains contain stem cell regions largely restricted to the forebrain 7,8, while the teleost zebrafish contains sixteen different stem cell domains and associated neurogenic zones in its entire brain 4,5,9,10. This variation between mammals and zebrafish might reflect a disparity in the mechanisms of stem cell maintenance and the neurogenic capacity of the progenitor cells. The lifelong competence of zebrafish to produce neurons in the brain could also pose clinical ramifications as the molecular mechanisms employed by zebrafish brain could be harnessed for therapeutic applications to tackle the neurodegenerative disorders in humans.
Several stem cells zones of the adult zebrafish brain have been analyzed and it has been shown that the cells lining the ventricular surface of those regions serve as progenitor cells 9,11-20. Detailed analyses in the zebrafish telencephalon, for example, identified the radial glial cells, which delineate the ventricular surface of this brain region to be neurogenic progenitors 19, 20. This holds true for other regions such as the cerebellum and the optic tectum, where ventricularly-located neuroepithelial cells provide the neurogenic input 16,21. To this end, understanding the molecular mechanisms governing the widespread neurogenic competency in adult zebrafish brain requires manipulation of gene expression in the progenitor cells.
Various methods were previously described for modulating gene function in zebrafish. These include generation of conditional transgenic lines expressing desired variants of a single protein, plasmid-based focal injections coupled to electroporation or chemical treatments. We previously designed a method for administering various substances including morpholino oligonucleotides or proteins into the adult zebrafish brain using cerebroventricular microinjection as a quick and efficient way of modulating gene function in the ventricular cells of the adult zebrafish forebrain 22. In our studies, vivo morpholinos were used because of their chemistry involving the morpholino molecule covalently linked to arginine rich delivery peptides, which enable efficient delivery to the cells of interest without need for extra permeabilization such as electroporation 23. This would allow a thorough targeting of the desired tissue after injection, just like what we observed in adult zebrafish telencephalon 22. Use of vivo morpholinos are therefore superior to already existing methods of tissue targeting such as electroporation or focal injection of DNA molecules.
Here, we demonstrate visually how we perform this operation and provide a step-by-step protocol. We start with explaining preparation of the injection mixture and the fish to be injected, and proceed by demonstrating how the injection apparatus is set up. We provide a description of the cerebroventricular injection method, which involves generating an incision in the skull and injection of the morpholinos using microinjector. We also elaborate on the critical points one needs to be cautious on during the whole procedure by stating them as optimization or troubleshooting guides.
Protocol
1. Preparation of the Injection Mixture
- Use vivo morpholinos as cells internalize them more efficiently than normal morpholino molecules. See the manufacturer’s information for details (Table 1: Reagents and Materials).
- Use a fluorescent tracking dye (e.g. CellTracker Red CMTPX, Invitrogen) to visualize the accuracy of the injection. This dye is metabolized in the cell and becomes fluorescent only in the cell after the uptake. Therefore, this step is crucial to determine the cells that are efficiently targeted by the cerebroventricular microinjection.
- Prepare phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10mM Na2HPO4, 2 mM KH2PO4, pH: 7.4) for diluting morpholino solution. If a dilution of the morpholino solution is required, always use PBS.
- Prepare 10 μl of the injection mix by adding 9 μl of the morpholino solution and 1 μl of the cell tracker dye (diluted or undiluted. The stock concentration of the morpholino solution is 500 μM. Our previous results 22 indicate that several different doses of the morpholino – ranging from 50 μM to 500 μM – result in different levels of knockdown from an efficient knockdown to hypomorphic phenotypes). 1 μl of solution is enough for injection into one zebrafish brain.
- Mix well and store at room temperature until the injection.
2. Preparation of the Injection Apparatus
- Prepare the glass injection capillaries using a needle puller. Use the following parameters: Heating cycle value: 463, pulling cycle value: 230, velocity: 1.5 sec, time: 75 msec.
- Turn on the pressure source and adjust the pressure settings to 50 psi or 3.5 bars.
- Adjust the microinjector settings as follows: hold pressure 20 psi, eject pressure 10 psi, period value of 2.5 and 100 msec range of gating.
- Turn on the ring illuminator and locate the ring over the microscope stage.
- Place the capillary holder to an appropriate position next to the microscope.
- Insert the glass capillary into the holder. Adjust the injection angle to 45 degrees.
- Snap off the tip of the glass capillary using a fine-end forceps and calibrate the pressure output from the orifice. This must be tested by immersing the tip of the needle into the fish water and giving a continuous pressure pulse. The air bubbles arising should be only one row, which is an indication of optimum pressure/orifice size. Please see the video for demonstration of this calibration.
- Load the glass capillary with the injection solution without trapping air bubbles.
- Apply pressure to remove the air between the tip of the glass capillary and the loaded injection solution.
3. Anesthesia
- Prepare the stock solution of the anesthetics (0.1% MESAB – ethyl-m-aminobenzoate methanesulphonate).
- Remove desired number of fish from their tanks into plastic mouse cages or other transport container with enough amount of water.
- Prepare the anaesthetization solution in a small plastic box by mixing 200 ml of fish water with 5 ml of anesthetics stock solution. Final concentration of the anesthetics: 0.0025% (v/v).
- Half-fill a plastic Petri dish with anesthetics. Use this dish for injections.
- Incubate the fish in the anesthetics until the fish subdues.
4. Incision and Cerebroventricular Microinjection
- Remove the fish from the box using a fish net and place it into the Petri dish.
- Gently hold the fish with the forceps and orient the head in a way that it tilts slightly downwards. This will facilitate the incision.
- Generate a small slit on the skull using a 30 gauge barbed-end needle. Use only the tip of the needle and do not penetrate more than enough into the brain as this will cause damage to the brain tissue and will manifest as bleeding.
- Keep holding the fish and insert the tip of the glass capillary through the incision site.
- Orient the tip of the glass capillary towards the telencephalon. Avoid touching the brain tissue as this creates tissue damage and also prevents dispersion of the injected solution.
- Inject the solution. The liquid disperses immediately after injection.
5. Recovery and Analyzing the Injection Accuracy
- Remove the fish from the injection setup and place into a plastic box with fresh fish water.
- Allow recovery.
- Re-anaesthetize the fish with 0.0025% MESAB briefly and check the fish under the fluorescence microscope. A thorough dispersion of red fluorescence is an indication of widespread distribution of the injected liquid.
- Put the fish back to the plastic box.
- Connect the plastic box to the circulation system to oxygenate the fish and maintain for longer periods of time.
- Use the fish for desired purposes such as behavioral analyses or sacrifice the animals at desired time points after the injection to prepare tissue specimens for further analyses (histological stainings, immunohistochemistry, etc.). The following references give examples of how to analyze the adult zebrafish brain: 9,11,17-20,24-27. The quickest time to observe an efficient knockdown (more than 50% knockdown) was 12 hr in our studies 22.
6. Suggested Scientific Controls
- Use a mismatch morpholino molecule that is not supposed to cause any phenotype as a negative control. Use either a standard morpholino molecule or specifically design morpholino controls for every gene of interest. Additionally, use the solvent (in this case PBS) as a further negative control to assess the non-specific effects or toxicity of the mismatch/control morpholino injections.
- Use PCNA morpholinos described previously 22 as positive controls. This morpholino causes efficient knockdown in the adult zebrafish brain (75-95% knockdown efficiency within 1 day of injection) 22.
Representative Results
Cerebroventricular microinjection leads to widespread distribution of the injected solution
Using the workflow and technical scheme of the cerebroventricular microinjection depicted in Figure 1, we administered morpholino oligonucleotides into the adult zebrafish brain. An accurate injection protocol leads to dispersion of the injected liquid throughout the brain and efficient targeting of the cells close to the ventricular surface (Figure 2A). Injections where the glass capillary impales the brain tissue will have a dense fluorescent signal at the point of injection (Figure 2B). If the injected amount is not enough, weak fluorescent signal will be observed (Figure 2C).
Cerebroventricular microinjection can be used for knocking down gene expression at the ventricular region of the adult zebrafish brain
We have shown that vivo morpholinos can penetrate a few cell diameters inside the ventricular surface and hence can efficiently target the ventricular cells (Figures 3A, 3B). Morpholino injection can knockdown gene expression as it will alleviate the production of the corresponding protein (an example is PCNA as shown in Figures 3C, 3D). We have previously demonstrated that knocking down genes using this technique leads to functional consequences during the adult neurogenesis (Figures 3E, 3F) or regeneration response (Figures 3G, 3H). We demonstrated that the efficient knockdown range (more than 50% knockdown) is attained approximately 12 hr after injection of the morpholinos 22. We also observed that efficient knockdown period is until approximately 5 days after injection for the PCNA protein 22. More than 70% knockdown was seen until 3 days after the injection 22. The knockdown curves are dependent on the level of expression of the endogenous proteins and the efficiency of the morpholino molecules; and has to be determined for every gene by the experimenter. In our studies, we did not see that the CVMI procedure compromises the survival of the animal, probably because the administration of the morpholino molecules is local and does not cause a systemic toxicity.
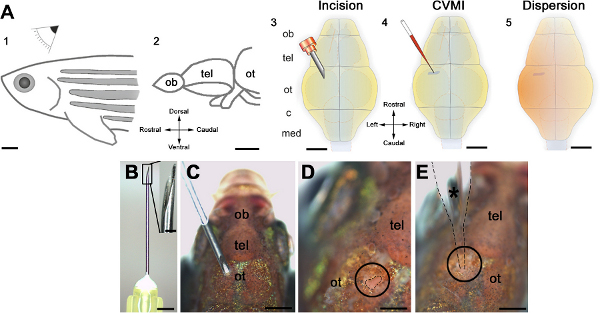
Figure 1. Schematic representation of the cerebroventricular injection technique. (A) The injection is performed from the dorsal of the adult zebrafish (1) at the level of optic tectum (ot, 2). An incision is made over the optic tectal skull plate with a 30-gauge needle (3). Using glass capillaries, the morpholino molecules are injected into the ventricular fluid (4). A thorough dispersion is achieved instantaneously (5). (B) The canula and its barbed-end is shown in high magnification. (C) The orientation of the needle before the incision. (D) The incision is circled and outlined by dashed lines. (E) The injection capillary is located as shown and injection is performed. All panels were adapted from ref. 22. Click here to view larger figure.
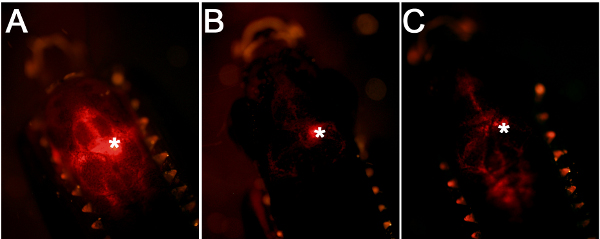
Figure 2. Comparison of injection efficiency and accuracy. (A) A good injection leads to targeting of the cells close to the ventricular surface. (B) If the glass capillary is inserted into the brain tissue, this will generate a centrally located fluorescence signal. (C) If the amount of injection is low, this will lead to weaker fluorescence signal.
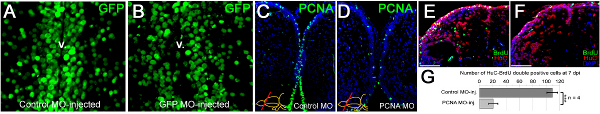
Figure 3. Morpholino penetration, efficiency of gene knockdowns and functional consequences. (A) GFP immunostaining on the telencephalic cross sections of control morpholino-injected Tg(H2A:GFP) transgenic line showing GFP-positive nuclei. (B) GFP immunostaining on the telencephalic cross sections of gfp antisense morpholino-injected Tg(H2A:GFP) transgenic line showing GFP-positive nuclei. Note the difference in the GFP-signal at the ventricular region. v: ventricle. (C) PCNA immunostaining on control morpholino-injected brains showing widespread distribution of PCNA-positive cells. (D) PCNA immunostaining on pcna antisense morpholino-injected brains showing significantly reduced amounts of PCNA-positive cells, showing that CVMI can knockdown endogenous genes. (E) BrdU and HuC/D immunostaining on control morpholino-injected brains to determine newborn neurons after a BrdU pulse-chase experiment described before 22. (F) BrdU and HuC/D immunostaining on pcna antisense morpholino-injected brains to determine newborn neurons after a BrdU pulse-chase experiment described before 22. (G) Knocking-down PCNA leads to significant reduction in generation of newborn neurons, indicating that CVMI can be used to knockdown endogenous genes, which give rise to functional consequences. All panels were adapted from ref. 22 Click here to view larger figure.
Discussion
The method we describe here allows easy and rapid administration of morpholinos into the adult zebrafish brain. We have demonstrated that our injection method efficiently blocks gene expression in the ventricular cells and results in functional consequences in the neurogenesis response.
There are important points to be cautious about while executing the cerebroventricular microinjection. For instance, the effect of the morpholino molecules depends on the concentration used. This concentration has to be determined by the end-user. We recommend starting with the stock solution (500 μM) and performing serial dilutions, and ideally to pre-test by injection into embryos using standard embryo injection protocols 28-30. Previously, we obtained different levels of knockdown efficiency with concentrations of morpholinos ranging from 50 μM to 500 μM 22. Second, the orifice of the glass capillary should not be large as this will lead to extensive liquid influx into the brain after the injection. Similarly, the opening must not be too narrow as this will prevent adequate injection. One can determine the optimum orifice-pressure combination by pumping air into a Petri dish with water. The bubbles arising should be in a single row but not in multiple rows. We demonstrate this in the video. Third, incubating the fish in the anesthetics is critical. The fish should not be kept longer than 2 min in the anesthetics. This will hamper the recovery rate after the injection. Fourth, the location of the incision is critical for thoroughly dispersing the injected liquid. The ventricular region over the optic tectum is larger above the midline and gets narrower laterally. Therefore, the experimenter should generate the slit in the skull close to the midline and just caudal to the skull plate covering the telencephalic region.
One of the advantages of the cerebroventricular injection (CVMI) method is its rapidness. CVMI is a quick method for assaying gene function. This feature is important and useful when compared to generation of transgenic lines for functional studies, which generally take several months. Additionally, CVMI leads to uniform distribution of the injected liquid and therefore provides a relatively thorough manipulation of gene activity, when compared to focal injections or electroporation. With CVMI, multiple genes can be knocked down simultaneously by preparing injection mixes containing multiple morpholinos oligonucleotide. CVMI can be used to inject different concentrations of a given morpholino oligonucleotides, and therefore can be used for analyzing hypomorphic phenotypes. Finally, this injection paradigm does not cause toxicity or compromise the survival of the animals.
The CVMI technique might be expanded for other type of studies such as injection of modulatory peptides, drugs, plasmid DNA or modulatory RNA molecules or other substances that might affect the physiology of the cells. Assaying combination of molecules and performing dose response analyses are possible using our method, allowing studying hypomorphic phenotypes. With these properties, CVMI proves to be a quick and easy assay for expression studies in the adult zebrafish brain, and opens up rapid screening and functional analyses.
The adult zebrafish brain can constitutively produce new neurons along the whole rostrocaudal axis and it can also regenerate after traumatic injuries. This is in stark contrast to mammalian brains with limited neurogenesis and if at all, rather poor regenerative capacity. Such widespread stem cell activity and recuperation ability makes zebrafish a useful model organism for understanding the molecular programs required for central nervous system regeneration, which are currently largely unknown. Therefore, investigating the molecular basis of the regenerative aptitude of zebrafish brain is an interesting realm of research that might explain the fundamental difference how fish and mammalian brains react after an injury, and also endow avenues for regenerative medical therapies in humans. In order to understand the molecular infrastructure of vertebrate brain plasticity and regeneration, glial cells serve as an important research area as they are the neurogenic progenitors 3,8,20. Thus, using CVMI technique to alter gene function in the radial glial cells of the zebrafish brain is instrumental in elucidating how fish brain can couple progenitor activity to efficient adult neurogenesis and regenerative response. We have recently shown the involvement and requirement of several factors and signaling pathways in the regenerative neurogenesis response of the adult zebrafish brain 24,26,27, and these studies were made possible by the use of CVMI method. Overall, the knowledge we gain from zebrafish brain could be harnessed to impose regenerative ability to the mammalian glial cells that react to injuries and will hence help designing clinical therapies for human neurological disorders and acute injuries.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 655) and the European Union (Zf-Health).
Materials
| Reagent/Material | |||
| CellTracker Red CMTPX | Life Technologies, Invitrogen | C34552 | Use at 1:100 dilution for measuring the injection accuracy |
| MESAB (ethyl-m-aminobenzoate methanesulphonate) | Sigma-Aldrich | A5040 | For anesthetizing the fish |
| Petri dishes | Sarstedt | 821,472 | For handling the fish during injection and imaging |
| Phosphate-buffered saline | Life Technologies, GIBCO | 10010-056 | As sterile dilution medium |
| Vivo morpholinos | Gene Tools Inc. | Customized | More info on vivo morpholinos: www.gene-tools.com |
| Equipment | |||
| Barbed-end needle | Becton-Dickinson | 305178 | To generate the incision in the skull |
| Dissecting microscope | Olympus, Leica, Zeiss | Varies with the manufacturer | Part of the whole injection setup |
| Dumont Tweezers | World Precision Instruments | 501985 | To snap off the tip of the glass capillary |
| Fluorescence camera | Zeiss, Nikon, Leica | Varies with the manufacturer | To visualize the fluorescence after injection |
| Gillies Dissecting Forceps | World Precision Instruments | 501265 | To hold the fish in position |
| Glass injection capillaries | World Precision Instruments | TWF10 | For microinjection |
| PicoNozzle kit (microinjector holder) | World Precision Instruments | 5430-12 | For microinjection |
| Pneumatic PicoPump (microinjector) | World Precision Instruments | SYS-PV820 | For microinjection |
| Ring illuminator; Ring Light Guide | Parkland Scientific | ILL-RLG | For illuminating the specimen |
References
- Stocum, D. . Regenerative Biology and Medicine. , (2006).
- Poss, K. D. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 11 (10), 710-722 (2010).
- Doetsch, F., Scharff, C. Challenges for brain repair: insights from adult neurogenesis in birds and mammals. Brain Behav. Evol. 58 (5), 306-322 (2001).
- Kaslin, J., et al. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 363 (1489), 101-122 (2008).
- Kizil, C., et al. Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 72 (3), 429-461 (2012).
- Grandel, H., Brand, M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev. Genes Evol. , (2013).
- Kempermann, G. . Adult Neurogenesis 2: Stem Cells and Neuronal Development in the Adult Brain. , (2010).
- Ihrie, R. A., Alvarez-Buylla, A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 331 (1), 179-191 (2008).
- Grandel, H., et al. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell. 295 (1), 263-277 (2006).
- Chapouton, P., et al. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 29 (8), 745-757 (2007).
- Adolf, B., et al. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 295 (1), 278-293 (2006).
- Byrd, C. A., Brunjes, P. C. Neurogenesis in the olfactory bulb of adult zebrafish. 神经科学. 105 (4), 793-801 (2001).
- Pellegrini, E., et al. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 501 (1), 150-167 (2007).
- Lam, C. S., et al. gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish. 238 (2), 475-486 (2009).
- Wang, X., et al. Identification of Wnt-responsive cells in the zebrafish hypothalamus. Zebrafish. 6 (1), 49-58 (2009).
- Kaslin, J., et al. Stem cells in the adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. J. Neurosci. 29 (19), 6142-6153 (2009).
- Ganz, J., et al. Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon. Glia. 58 (11), 1345-1363 (2010).
- März, M., et al. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia. 58 (7), 870-888 (2010).
- Rothenaigner, I., et al. Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development. 138 (8), 1459-1469 (2011).
- Kroehne, V., et al. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 138 (22), 4831-4841 (2011).
- Ito, Y., et al. Characterization of neural stem cells and their progeny in the adult zebrafish optic tectum. Dev. Biol. 342 (1), 26-38 (2010).
- Kizil, C., Brand, M. Cerebroventricular microinjection (CVMI) into adult zebrafish brain is an efficient misexpression method for forebrain ventricular cells. PLoS One. 6 (11), e27395 (2011).
- Morcos, P. A., et al. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. Biotechniques. 45 (6), 613-614 (2008).
- Kizil, C., et al. The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev. 7, 27 (2012).
- Chapouton, P., et al. her5 expression reveals a pool of neural stem cells in the adult zebrafish midbrain. Development. 133 (21), 4293-4303 (2006).
- Kizil, C., et al. Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Developmental Cell. 23 (6), 1230-1237 (2012).
- Kyritsis, N., et al. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 338 (6112), 1353-1356 (2012).
- Rosen, J. N., et al. Microinjection of zebrafish embryos to analyze gene function. J. Vis. Exp. (25), e1115 (2009).
- Yuan, S., Sun, Z. Microinjection of mRNA and morpholino antisense oligonucleotides in zebrafish embryos. J. Vis. Exp. (27), e1113 (2009).
- Rikin, A., et al. A reverse genetic approach to test functional redundancy during embryogenesis. J. Vis. Exp. (42), e2020 (2010).

