Recording of Local Field Potential in Mouse Hippocampal-Entorhinal Cortex Slices
Abstract
Source: Whitebirch, A. C. Acute Mouse Brain Slicing to Investigate Spontaneous Hippocampal Network Activity. J. Vis. Exp. (2020)
The video demonstrates a procedure to record the local field potential (LFP) in mouse hippocampal-entorhinal cortex slices. Electrical stimulation is provided at the CA3 stratum radiatum of the hippocampus, which causes changes in the postsynaptic potential at the CA1 stratum pyramidale. The combined change in the membrane potential of CA1 neurons, termed the local field potential, is recorded.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Transcardial perfusion
- Suspend a 60 mL capacity syringe as a reservoir for sucrose cutting solution approximately 18 inches above the benchtop (e.g., using a three-pronged swivel clamp on a vertical post). Attach tubing to the bottom of the reservoir, run the tubing through a roller clamp, and connect the other end of the tube to a clean 20 G needle.
- Fill the 60 mL reservoir with approximately 30 mL of chilled sucrose solution and direct a carbogen line into the sucrose reservoir to continually bubble the perfusate.
NOTE: Ensure that the sucrose is flowing through the tubing and needle without any trapped bubbles. The flow rate should be just fast enough to have a steady, continual stream of dripping sucrose from the needle. - Using a blender, crush and blend the frozen sucrose into an icy slurry and use the large spatula/spoon to distribute the slurry.
- Place a small amount of sucrose slurry around the edges of the glass Petri dish lid. Add a small amount of sucrose slurry to the Petri dish bottom.
- Add roughly 20–30 mL of sucrose slurry to the perfusion fluid reservoir, mixing it well with the 30 mL of chilled liquid sucrose until the reservoir contains a mixture of very cold, predominantly liquid sucrose with some remnant frozen solution.
- Place the mouse into a chamber connected to an isoflurane vaporizer. With oxygen flowing at approximately 2 L/min, turn the dial of the vaporizer to deliver isoflurane at 5% concentration and start a timer.
NOTE: Keep this timer going throughout the course of slicing to gauge how quickly the slices are obtained. The procedure should be done as quickly as possible, such that slicing is complete, and tissue is recovering in the interface chamber within 15–20 min of the animal entering the isoflurane chamber. Watch the mouse to ensure a state of deep anesthesia is achieved. After a minimum of 5 min, the mouse should be deeply anesthetized and unresponsive to toe pinches. - Immediately before removing the mouse from the isoflurane chamber, fill the Petri dish lid with chilled sucrose solution to a depth of approximately 3–5 mm and fill the Petri dish bottom with chilled sucrose solution to a depth of approximately 1.0 cm.
- Quickly transfer the mouse to the left absorbent pad and use the 3 pieces of tape to secure the forelimbs and tail. Perform a hindlimb toe pinch to ensure that the mouse is unresponsive before any incision is performed. Using the large tissue forceps and surgical scissors, tent the skin and make a lengthwise incision from the bottom of the sternum to the top of the chest. Using the forceps pull up on the sternum and use the scissors to cut through the diaphragm.
NOTE: The initial positioning of the mouse and first several incisions to sever the diaphragm should be performed as quickly as possible to ensure the mouse does not regain consciousness. To ensure an adequate depth of anesthesia throughout the procedure, a nose cone can be placed on the mouse to deliver isoflurane during the perfusion. - Use the scissors to cut through the rib cage on each side, cutting in one large motion towards the point where the forelimb meets the body. With the forceps, swing the front of the rib cage away and up toward the head, and then fully remove it with a horizontal cut using large scissors. Hold the heart in place using the large forceps and insert the 20 G perfusion needle into the left ventricle. Once the needle is positioned correctly, the left side of the heart should quickly pale as chilled sucrose solution fills the ventricle.
- Use the small dissector scissors to cut into the right atrium and allow the blood to flow out of the circulatory system. If the incisions are performed correctly, there should be minimal damage to the heart, and it should continue pumping throughout the perfusion.
NOTE: Rolled-up pieces of tissue paper can be used to wick away blood and sucrose solution from the heart and maintain visibility of the perfusion needle during the procedure. - Ensure that the perfusion needle stays in place and does not fall out of the left ventricle. With a proper flow rate and placement, the liver will start to pale to a light tan/beige color with 20–30 s.
- After 30–45 s, use the large scissors to decapitate the mouse. Holding the head in the left hand, push or peel the skin away from the skull. In a well-perfused animal, the skull should be very pale, and the brain should appear a very light pink color (approaching white) through the skull without clearly visible blood vessels.
2. Extract the brain and cut slices
- Using the small Bonn scissors, make two lateral cuts through the skull towards the midline at the front of the skull, near the eyes. Make two additional cuts on either side of the base of the skull.
- Transfer the head to the bottom of the glass Petri dish where it should be mostly immersed in chilled sucrose solution. Use the small Bonn scissors to cut down the midline along the length of the skull, pulling up with the scissors to minimize damage to the underlying brain tissue. Using the small tissue forceps, firmly grasp each side of the skull and swing it up and away from the brain, like opening a book.
- Use the fingers of the left hand to hold the flaps of skull open and insert the microspatula under the brain near the olfactory bulbs. Flip the brain out of the skull into the sucrose and use the microspatula to sever the brain stem. Wash the brain to remove any residual blood, fur, or other tissues.
- Use the large spatula/spoon to transfer the brain to the lid of the glass Petri dish. With the other half of the double-edged razor blade previously set aside, make two coronal cuts to first remove the cerebellum and then the most anterior portion of the brain, including the olfactory bulbs (Figure 1).
- Apply cyanoacrylate adhesive to the agar ramp. Very briefly place the brain onto a piece of dry filter paper using the large tissue forceps and then immediately transfer it to the agar ramp, placing the ventral surface of the brain onto the adhesive.
NOTE: For slices of the intermediate hippocampus, the brain should be oriented with the anterior facing up the slope of the ramp, and the posterior closer to the blade. For slices of the ventral hippocampus, orient the brain with the anterior end pointed down the slope of the ramp, with the posterior end at the top of the ramp, further away from the blade. In either case, the brain should be positioned at the top of the ramp, such that the coronally-cut surface of the brain contacts the agar backing block (Figure 1). - Place the slicing platform with the agar and brain into the slicing chamber of the microtome and completely immerse with chilled sucrose solution. Use the large spatula/spoon to transfer some sucrose slurry to the chamber, stirring to melt any frozen sucrose and rapidly bring down the temperature of the mixture to ~1–2 °C.
NOTE: Watch the temperature gauge throughout the slicing. If the sucrose solution warms above 3 °C, add more slurry and mix to bring the temperature back down. - Cut slices at a thickness of 450 μm with the microtome speed set to 0.07 mm/s. As each slice is freed, use the small tissue forceps and a sharp scalpel to first separate the two hemispheres, and then to cut away tissue until the slice consists primarily of the hippocampus and parahippocampal regions (Figure 1).
- Use a plastic transfer pipette to transfer the slices individually to the interface recovery chamber, ensuring that they are positioned at the interface of the ACSF and the air with only a thin meniscus of artificial cerebrospinal fluid (ACSF) covering the slices. Tightly close the lid of the chamber and allow slices to recover at 32 °C for 30 min.
- After the initial recovery at 32 °C, bring the interface recovery chamber out of the water bath and place on a stirrer set to a slow speed such that the magnetic stir bar promotes circulation of ACSF within the chamber. Allow it to gradually cool to room temperature as the slices recover for an additional 90 min. Ensure that the recovery chamber is continually bubbled with carbogen and do not allow large bubbles to become trapped underneath the slices.
3. Perform local field potential (LFP) recordings of spontaneous activity
- Prepare for LFP recordings by turning on all necessary equipment, including the computer running the acquisition software, the monitor connected to the computer, the stimulators, the micromanipulator, the temperature controller, the microscope light source, the microscope-attached camera, the microelectrode amplifier, the digitizer, and the peristaltic pump. If using a central vacuum system, open the wall valve to prepare the vacuum line that will remove ACSF from the recording chamber.
- Fill the heated reservoir with ACSF, then place one end of the tubing into the 400 mL beaker containing the bubbling ACSF. Turn on the peristaltic pump to direct ACSF from the 400 mL beaker to the heated reservoir, and from the reservoir onward to the recording chamber. Tap or pinch the tubing to release any trapped bubbles.
- Adjust the peristaltic pump to ensure that the ACSF flow rate through the recording chamber is fast (~ 8–10 mL/min). Use a temperature probe to ensure that the ACSF is 32 °C in the center of the recording chamber.
NOTE: If ACSF is delivered to the recording chamber using a peristaltic pump, a high flow rate can result in a significant fluctuation in flow rate. Consistent flow rates can be achieved using a simple pulsation dampener consisting of a series of empty syringes integrated into the tubing (Figure 2). - Prepare stimulation and recording pipettes from borosilicate glass capillaries using a heated filament puller. The puller protocol should be configured to yield pipettes with a resistance of 2–3 MOhm for stimulation or local field potential recording electrodes.
- Fill stimulation pipettes with 1 M NaCl and LFP pipettes with ACSF.
- Briefly clamp the tubing and turn off the pump to pause the flow of ACSF. Transfer a slice to the recording chamber using fine forceps to grasp a corner of the lens paper the slice is resting on-the slice will stick to the lens paper. Place the lens paper and slice into the recording chamber with the slice facing down, then “peel” away the lens paper leaving the slice submerged in the recording chamber. Secure the slice using a harp.
NOTE: Harps can be purchased pre-made or made in-lab using a U-shaped piece of stainless steel or platinum and fine nylon filament. - Place a NaCl-filled stimulation pipette in the manual micromanipulator and slowly advance the tip of the pipette into the surface of the slice (e.g., in the stratum radiatum layer) at an angle of approximately 30–45°. Once the tip of the pipette enters the tissue, advance the pipette forward slowly and refrain from large movements in the lateral or vertical direction that could unnecessarily damage axons within the slice. Place the tip of the pipette at least 50–100 μm deep into the slice to avoid cells near the surface that were damaged during slicing.
- Place an ACSF-filled LFP pipette into the pipette holder attached to the motorized micromanipulator. Apply a very light positive pressure using either mouth pressure or a 1 mL syringe connected via a stopcock valve and a short length of tubing to the pipette holder.
NOTE: Position LFP pipettes within the slice in order to record the signals of interest: in the case of sharp wave ripples, one LFP pipette should be placed in the stratum pyramidale (SP) and a second pipette in the stratum radiatum (SR). This configuration allows for simultaneous recordings of the negative sharp-wave in the SR and the high-frequency ripple oscillation in the SP (Figure 3). - Using the micromanipulator, slowly advance the tip of the LFP pipette into the region of interest (e.g., the CA1 pyramidal cell layer) at an angle of approximately 30–45°. Place the tip of the pipette at least 50–100 μm deep into the slice to avoid cells near the surface that were damaged during slicing. While advancing the LFP pipette, continually deliver a small voltage test pulse using the acquisition software and watch for a sudden increase in electrode resistance. This may indicate that the pipette has been clogged or pressed up against a cell.
- Once the LFP pipette is positioned in the region of interest, carefully release the positive pressure by opening the valve on the tubing attached to the pipette holder.
- To record the LFP in a second location simultaneously, repeat steps 6.8–6.10 with a second pipette and micromanipulator.
- Use the microelectrode amplifier in the current clamp configuration to record spontaneous activity in the local field potential after using the bridge balance function in the acquisition software to correct for series resistance of the pipette. Spontaneous sharp-wave ripples (SWRs) will appear as positive deflections in the extracellular potential of the SP layer (Figure 3).
- In order to record evoked field potentials, use the stimulators connected to the digitizer to deliver a short (200 us) square voltage pulse through the NaCl-filled stimulation pipette. Adjust the stimulation voltage dial to evoke a range of response amplitudes.
Representative Results
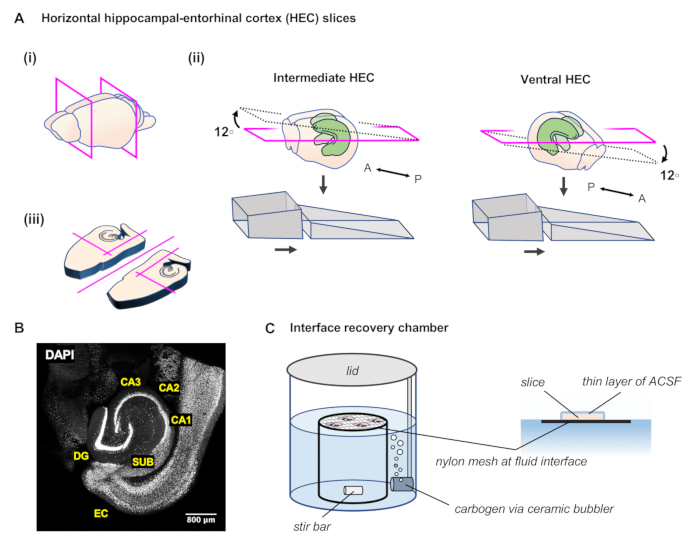
Figure 1: Preparation of horizontal angled hippocampal-entorhinal cortex (HEC) slices. (A)(i) After extracting the brain, perform two coronal cuts with a razor blade to remove the posterior and anterior portions of the brain. (ii) The agar ramp is formed of two angled portions glued to the microtome slicing platform. To prepare slices of the intermediate hippocampus, place the brain block onto the agar ramp with the anterior surface facing up the slope and making contact with the tall backing portion of the ramp. To prepare slices of more ventral hippocampus, place the brain block onto the agar ramp with the anterior surface facing down the slope, so that the posterior cut surface makes contact with the tall backing portion of the ramp. (iii) As each slice is freed, perform several more cuts with the scalpel to separate the hemispheres and remove unnecessary tissue. (B) Representative image of the resulting slice with cell nuclei labeled by DAPI. (C) In an interface recovery chamber, slices are placed on pieces of lens paper on top of a stainless steel or nylon mesh, level with the surface of the ACSF. A ceramic bubbler conveys carbogen into the chamber and a magnetic stir bar continually mixes the fluid in the chamber. A thin film of ACSF covers the top surface of the slice, enhancing diffusion of oxygen from the humid carbogen-rich air of the chamber.
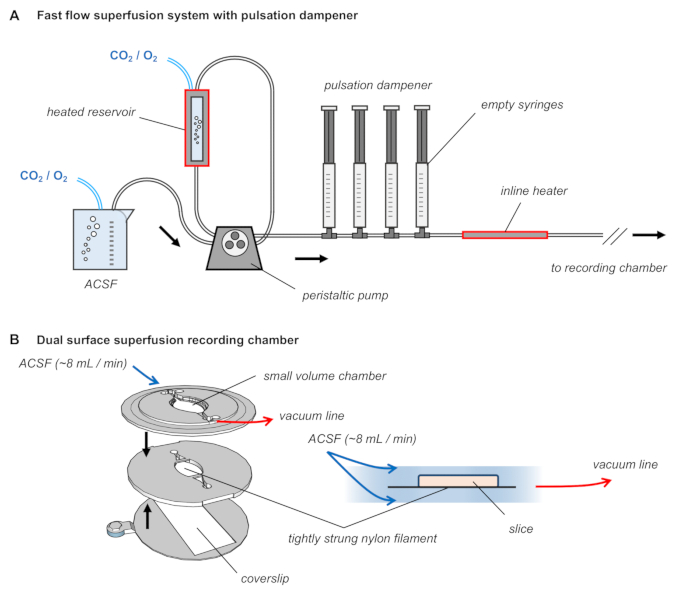
Figure 2: Dual surface superfusion recording chamber with pulsation dampener in the ACSF delivery tubing. (A) Diagram of the superfusion system. ACSF is warmed to 32 ˚C, constantly bubbled with carbogen gas, and delivered at approximately 8–10 mL/min using a peristaltic pump with a pulsation dampener consisting of a series of air-filled syringes. (B) The recording chamber consists of three 3D-printed layers, the middle of which is strung with nylon filament. The slice rests upon this strung filament and ACSF flows above and below the tissue.
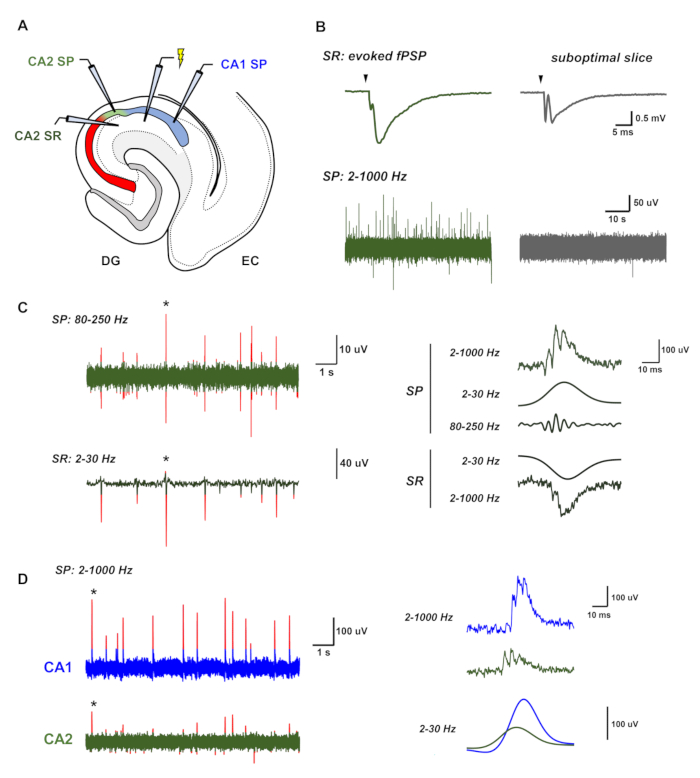
Figure 3: Representative recordings of spontaneous sharp-wave ripples from HEC slices. (A) A simplified diagram of the HEC slice showing the positions of the recording and stimulation electrodes. (B) Representative recordings of LFP activity from both an active, healthy slice and a suboptimal slice. The healthy slice (left, in green) shows large evoked field responses and spontaneous sharp-wave ripples (SWRs), visible as irregularly occurring positive deflections in the local field potential of the SP layer. In contrast, an unhealthy slice shows small, evoked field responses and no spontaneous activity (right, in gray). (C) Representative recordings of SWRs in the CA2 region, consisting of a negative deflection in the LFP in the SR layer and a high frequency oscillation with an underlying positive deflection in the LFP in the SP layer. Peaks in each channel greater than three standard deviations of the signal amplitude are highlighted in red. A bandpass filter of 2–30 Hz isolates the underlying positive and negative envelope of the sharp wave in the SP and SR layer, respectively, while a bandpass filter of 80–250 Hz is used to isolate the high-frequency oscillation of the ripple in the SP layer. (D) SWRs in vitro propagate from CA2/CA3 to CA1. In these representative recordings, SWRs in CA2 (green, bottom) precede that in CA1 (blue, top) by several milliseconds. Peaks in each channel greater than three standard deviations of the signal amplitude are highlighted in red.
Disclosures
The authors have nothing to disclose.
Materials
| Acute brain slice incubation holder | NIH 3D Print Exchange | 3DPX-001623 | Designed by ChiaMing Lee, available at https://3dprint.nih.gov/discover/3dpx-001623 |
| Adenosine 5′-triphosphate magnesium salt | Sigma Aldrich | A9187-500MG | |
| Ag-Cl ground pellets | Warner | 64-1309, (E205) | |
| agar | Becton, Dickinson | 214530-500g | |
| ascorbic acid | Alfa Aesar | 36237 | |
| beaker (250 mL) | Kimax | 14000-250 | |
| beaker (400 mL) | Kimax | 14000-400 | |
| biocytin | Sigma Aldrich | B4261 | |
| blender | Oster | BRLY07-B00-NP0 | |
| Bonn scissors, small | becton, Dickinson | 14184-09 | |
| borosilicate glass capillaries with filament (O.D. 1.5 mm, I.D. 0.86 mm, length 10 cm) | Sutter Instruments | BF150-86-10HP | Fire polished capillaries are preferable. |
| calcium chloride solution (1 M) | G-Biosciences | R040 | |
| camera | Olympus | OLY-150 | |
| compressed carbogen gas (95% oxygen / 5% carbon dioxide) | Airgas | X02OX95C2003102 | |
| compressed oxygen | Airgas | OX 200 | |
| constant voltage isolated stimulator | Digitimer Ltd. | DS2A-Mk.II | |
| coverslips (22×50 mm) | VWR | 16004-314 | |
| cyanoacrylate adhesive | Krazy Glue | KG925 | Ideally use the brush-on form for precision |
| data acquisition software | Axograph | N/A | Any equivalent software (e.g. pClamp) would work. |
| Dell Precision T1500 Tower Workstation Desktop | Dell | N/A | Catalog number will depend on specific computer – any computer will work as long as it can run electrophysiology acquisition software. |
| Digidata 1440A | Molecular Devices | 1-2950-0367 | |
| digital timer | VWR | 62344-641 | 4-channel Traceable timer |
| disposable absorbant pads | VWR | 56616-018 | |
| dissector scissors | Fine Science Tools | 14082-09 | |
| double-edge razor blades | Personna | BP9020 | |
| dual automatic temperature controller | Warner Instrument Corporation | TC-344B | |
| dual-surface or laminar-flow optimized recording chamber | N/A | N/A | The chamber presented in this protocol is custom made. A commercial equivalent would be the RC-27L from Warner Instruments. |
| equipment rack | Automate Scientific | FR-EQ70" | A rack is not strictly necessary but useful for organizing electrophysiology |
| Ethylene glycol-bis(2-aminoethyiether)- N,N,N',N'-teetraacetic acid (EGTA) | Sigma Aldrich | 324626-25GM | |
| filter paper | Whatman | 1004 070 | |
| fine scale | Mettler Toledo | XS204DR | |
| Flaming/Brown micropipette puller | Sutter Instruments | P-97 | |
| glass petri dish (100 x 15 mm) | Corning | 3160-101 | |
| glucose | Fisher Scientific | D16-1 | |
| Guanosine 5′-triphosphate sodium salt hydrate | Sigma Aldrich | G8877-250MG | |
| ice buckets | Sigma Aldrich | BAM168072002-1EA | |
| isoflurane vaporizer | General Anesthetic Services | Tec 3 | |
| lab tape | Fisher Scientific | 15-901-10R | |
| lens paper | Fisher Scientific | 11-996 | |
| light source | Olympus | TH4-100 | |
| magnesium chloride solution (1 M) | Quality Biological | 351-033-721EA | |
| magnetic stir bars | Fisher Scientific | 14-513-56 | Catalog number will be dependent on the size of the stir bar. |
| micromanipulator | Luigs & Neumann | SM-5 | |
| micromanipulator (manual) | Scientifica | LBM-2000-00 | |
| microscope | Olympus | BX51WI | |
| microspatula | Fine Science Tools | 10089-11 | |
| monitor | Dell | 2007FPb | |
| MultiClamp 700B Microelectrode Amplifier | Molecular Devices | MULTICLAMP 700B | The MultiClamp 700B should include headstages, pipette holders, and a model cell. |
| N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid), (HEPES) | Sigma Aldrich | H3375-25G | |
| needle (20 gauge, 1.5 in length) | Becton, Dickinson | 305176 | |
| nylon filament | YLI Wonder Invisible Thread | 212-15-004 | size 0.004. This cat. # is from Amazon.com |
| nylon mesh | Warner Instruments Corporation | 64-0198 | |
| perstaltic pump | Harvard Apparatus | 70-2027 | |
| Phosphocreatine di(tris) salt | Sigma Aldrich | P1937-1G | |
| pipette holders | Molecular Devices | 1-HL-U | |
| platinum wire | World Precision | PT0203 | |
| polylactic acid (PLA) filament | Ultimaker | RAL 9010 | |
| potassium chloride | Sigma Aldrich | P3911-500G | |
| potassium gluconate | Sigma Aldrich | 1550001-200MG | |
| potassium hydroxide | Sigma Aldrich | 60377-1KG | |
| razor blades | VWR | 55411-050 | |
| roller clamp | World Precision Instruments | 14041 | |
| scale | Mettler Toledo | PM2000 | |
| scalpel handle | Fine Science Tools | 10004-13 | |
| slice harp | Warner | SHD-26GH/2 | |
| sodium bicarbonate | Fisher Chemical | S233-500 | |
| sodium chloride | Sigma Aldrich | S9888-1KG | |
| sodium phosphate monobasic anhydrous | Fisher Chemical | S369-500 | |
| sodium pyruvate | Fisher Chemical | BP356-100 | |
| spatula | VWR | 82027-520 | |
| spatula/spoon, large | VWR | 470149-442 | |
| sterile scalpel blades | Feather | 72044-10 | |
| stirrer / hot plate | Corning | 6795-220 | |
| stopcock valves, 1-way | World Precision Instruments | 14054 | |
| stopcock valves, 3-way | World Precision Instruments | 14036 | |
| sucrose | Acros Organics | AC177142500 | |
| support for swivel clamps | Fisher Scientific | 14-679Q | |
| surgical scissors, sharp/blunt | Fine Science Tools | 14001-12 | |
| syringe (1 mL) | Becton, Dickinson | 309659 | |
| syringe (60 mL with Luer-Lok tip) | Becton, Dickinson | 309653 | |
| three-pronged clamp | Fisher Scientific | 05-769-8Q | |
| tissue forceps, large | Fine Science Tools | 11021-15 | |
| tissue forceps, small | Fine Science Tools | 11023-10 | |
| transfer pipettes | Fisher Scientific | 13-711-7M | |
| tubing | Tygon | E-3603 | ID 1/16 inch, OD 3/16 inch |
| tubing | Tygon | R-3603 | ID 1/8 inch, OD 1/4 inch |
| vacuum grease | Dow Corning | 14-635-5D | |
| vibrating blade microtome | Leica | VT 1200S | |
| vibration-dampening table with faraday cage | Micro-G / TMC-ametek | 2536-516-4-30PE | |
| volumetric flask (1 L) | Kimax | KIM-28014-1000 | |
| volumetric flask (2 L) | PYREX | 65640-2000 | |
| warm water bath | VWR | 1209 |

