Calcium Imaging in Individual Neurons Regulating Egg-Laying Behavior in Caenorhabditis elegans
Abstract
Source: Ravi, B. et al., Ratiometric Calcium Imaging of Individual Neurons in Behaving Caenorhabditis Elegans. J. Vis. Exp. (2018).
This video demonstrates a technique for calcium imaging in individual neurons of behaving Caenorhabditis elegans using genetically encoded fluorescent reporter proteins. It outlines the steps involved in imaging worm movement and measuring reporter fluorescence ratios from neurons to correlate intracellular calcium levels with neuronal activity and behavioral changes during egg-laying.
Protocol
1. Strains, Culture Media, and Mounting of Animals
- Grow C. elegans worms at 20 °C on standard 60-mm Nematode Growth Medium (NGM) agar plates seeded with OP50 E. coli bacterial food.
- Prepare two plasmids for each cell-specific promoter of interest: one, driving expression of GCaMP5 to record intracellular Ca2+, and the second, driving expression of mCherry to allow for ratiometric quantitation of GCaMP5 fluorescence changes and simplify object finding and measurement.
NOTE: GCaMP5:mCherry ratiometric imaging corrects for fluctuations in GCaMP5 fluorescence that result from changes in focus and animal movement, not actual changes in intracellular Ca2+. - Inject GCaMP5- and mCherry-expressing plasmids along with the pL15EK visible rescue marker plasmid into the gonads of LX1832 lite-1(ce314), lin-15(n765ts) X mutant animals and recover non-Muv lin-15(+) animals expressing GCaMP5 and mCherry from a high-copy transgene. Use the lite-1 mutant background to reduce blue-light avoidance behavior.
- Integrate transgenes to chromosomes to reduce mosaicism and simplify the generation of compound mutant strains.
NOTE: The LX2004 strain described here carries an integrated high-copy transgene that expresses GCaMP5 and mCherry in the HSNs from the nlp-3 promoter (see Table of Materials). The nlp-3 promoter was found to drive strong expression in the HSNs of late L4 and adult animals without causing significant defects in HSN development or egg-laying behavior compared to other promoters tested (e.g.,tph-1, egl-6, and unc-86). This strain and others that express GCaMP5 and mCherry in the ventral cord (VC) motor neurons, vulval muscles, and uv1 neuroendocrine cells are available from the Caenorhabditis Genetics Center, and details of their construction have been described. - Apply OP50 bacterial food from a seeded NGM agar plate to the bottom of a platinum worm pick and use it to transfer ~ 20 late L4 LX2004 animals that express GCaMP5 and mCherry in the HSNs from the nlp-3 promoter. Ensure that the developing vulva appears in a stereomicroscope as a clear dark spot surrounded by a white crescent. Incubate animals at 20 °C for 24-40 h.
- After the incubation, apply OP50 to the pick and transfer ~ 3 of the worms to an unseeded NGM plate, leaving a small amount of food behind for the worms to feed upon during imaging. Ensure sufficient food, as too little bacterial food will encourage the worms to wander away from the center of the plate, while too much food will increase background fluorescence and cause hypoxia.
- Use a flat rounded spatula to cut a ~ 20 mm x 20 mm chunk from the plate carrying the worms and transfer the chunk face-down onto the center of a clean 24 mm x 60 mm #1.5 coverslip (Figure 1). Start the application from one side to keep bubbles from being trapped under the coverslip and interfering with imaging. Apply a 22 mm x 22 mm #1 coverslip to the top of the chunk to reduce sticking and evaporation.
2. Hardware and Instrumentation Setup
- Place the staged, transgenic worms onto the stage of an inverted microscope with a ≥ 0.7 Numerical Aperture Plan-Apochromat 20x objective, motorized XY stage controllable with a joystick, an image splitter, and fluorescence filters for simultaneous GCaMP5 and mCherry excitation and emission, cameras for fluorescence and infrared brightfield imaging, and a triggerable Light Emitting Diode (LED) illumination system (Figure 2A).
NOTE: An 80/20 beam-splitter sends 80% of the image signal to the fluorescence camera and 20% to the brightfield camera. An upright microscope can also be used, but the NGM chunk should be placed in between a glass slide and the large coverslip in this case.- Use the binocular eyepieces to select a worm for imaging. When an animal is selected, slide the infrared filter in place above the condenser.
- Transistor-transistor logic (TTL) triggers
- Attach coaxial cables from each of the three TTL trigger output lines on the fluorescence camera. Connect the first output to the BNC input #3 of the LED illumination system.
- Connect the second output to a BNC 'banana' adapter with green and brown wires from the 8-pin GPIO connector running to GPIO input #3 (pin 4) and ground (pin 5) of the infrared camera, respectively.
- Connect the third output to a BNC 'banana' adapter with jumper wires running to the #8 digital input and ground in the digital acquisition device (Figure 2B).
- Digital acquisition device (DAQ)
- Connect the DAQ microcontroller board (see Table of Materials) to the PC via a USB cable. Update the firmware with the standard Firmata protocol (see Table of Materials) and configure the USB port to communicate at 57600 baud.
- LEDs
- Run the LED Controller software (see Table of Materials). Switch the Trigger Mode from 'Continuous' to 'Gated' and select Trigger Channel '3' for both the 470 and 590 nm LEDs.
- Turn on and enter LED power for each LED (e.g., set the 470 nm LED to 20% and the 590 nm LED to 40%).
- Run the serial-stage communication script 'XY-stage-final' in Bonsai (see Table of Materials). Click on the grey 'CsvWriter' node and select a folder to save the recorded X and Y stage information (Figure 3). Press the green arrowhead in the toolbar to initialize the DAQ.
NOTE: The script will start recording the X and Y stage position when the fluorescence camera sends a TTL signal. This script outputs four columns of data: frame number, X position (µm), Y position (µm), and the interval between frames (s). - In the infrared camera recording software (see Table of Materials), under 'Custom Video Modes,' select "Mode 1" (2 x 2 binning; 1,024 x 1,024 pixels), and "Pixel Format Raw 8". Under 'Trigger / Strobe', set the trigger input line (GPIO) to "3", the polarity to "High", and the 'Mode' to "14". Toggle 'Enable' to stop frame acquisition until a TTL trigger signal is received.
- Leaving that window open, click the red "Record" button on the main camera view toolbar. Select the folder for image sequences to be saved. Select 'Buffered' Recording mode and save 'Images' in JPEG format. Click "Start Recording" to initialize the acquisition.
- Fluorescence camera and image splitter
NOTE: GCaMP5 and mCherry fluorescence channels must be collected simultaneously to ensure proper image registration for ratiometric quantitation. An image splitter allows two-channel acquisition of the GCaMP5 and mCherry fluorescence onto one sensor.- In the 'Capture' tab of the image acquisition software (see Table of Materials), set exposure time to 10 ms, binning to 4x, and image depth to 16-bit. Select a centered camera subarray of 512 pixels wide by 256 pixels high. Click 'Show Output Trigger Options' and set all Triggers to 'Positive.'
NOTE: The DAQ can occasionally miss TTL triggers if they are 10 ms or less. - Ensure that 'Trigger 1' and 'Trigger 2' are set to 'Exposure' while 'Trigger 3' is set to 'Programmable' with a 'Period' of 25 ms. Under the 'Sequence' tab, select 'Time Lapse' with a 'Field Delay1' of 50 ms to collect images at 20 Hz. Select 'Save to Temporary Buffer.'
- In the 'Capture' tab of the image acquisition software (see Table of Materials), set exposure time to 10 ms, binning to 4x, and image depth to 16-bit. Select a centered camera subarray of 512 pixels wide by 256 pixels high. Click 'Show Output Trigger Options' and set all Triggers to 'Positive.'
- PMT gain and laser intensity during three channel confocal imaging
- Set the PMT gain so the background fluorescence is at a level just above the minimum (black level). Increase 561 nm (green) laser power until a single saturated 12-bit or 16-bit pixel is observed at the presynaptic terminus in the mCherry channel.
- Adjust 488 nm (blue) laser intensity so that GCaMP5 fluorescence is just visible at the presynaptic terminus above the background. This low setting prevents the saturation of the GCaMP5 pixels when the fluorescence increases in response to strong Ca2+ transients. Open the confocal pinhole all the way to maximize light capture.
3. Ratiometric Ca2+ Imaging and Behavior Recording
- Under the 'Sequence' tab in the fluorescence acquisition software, click "Start" to begin recording. Track the worm with the joystick, keeping the cell(s) and synapses of interest in focus and in the center of the field of view (FOV). Click the 'Stats' button in the histogram window to show pixel statistics of each channel.
- Adjust the LED power to ensure a maximum single-pixel mCherry fluorescence at the presynaptic terminus of ≥ 8,000 counts (~ 4,000 photoelectrons), giving a ~ 12-bit dynamic range above the background (~ 100 photoelectrons). GCaMP5 signals at the presynaptic terminus during resting (low) Ca2+ should be around ~ 2,500 counts – just visible above background.
- Record until one egg-laying active state is reached; typically, this occurs every 20-30 min in a wild-type worm. Save a 10-min subset (12,001 frames), 6,000 frames before and after the first egg-laying event (frame 6,001). Be sure to keep the same subset of brightfield images of worm behavior and timepoints of XY stage position, or the precise synchronization of data streams will be lost.
- Use ImageJ and the BioFormats plugin (see Table of Materials) to convert image sequences to Image Cytometry Standard (.ics) format so that it can be imported into the Ratiometric Quantitation software.
Representative Results
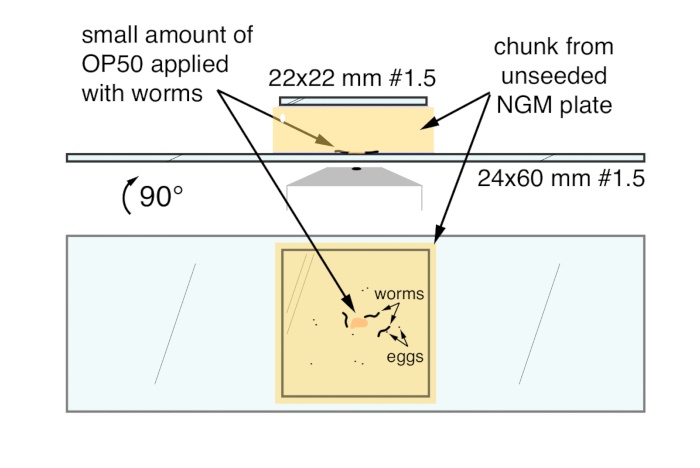
Figure 1. C. elegans mounting technique for high-resolution imaging of egg-laying circuit activity and behavior. Top, the final mount from the side. Bottom, the final mount as viewed through the bottom of the large coverslip. Arrows indicate OP50 bacterial food, C. elegans worms, and eggs, sandwiched between the NGM agar chunk and the large 24 mm x 60 mm coverslip.
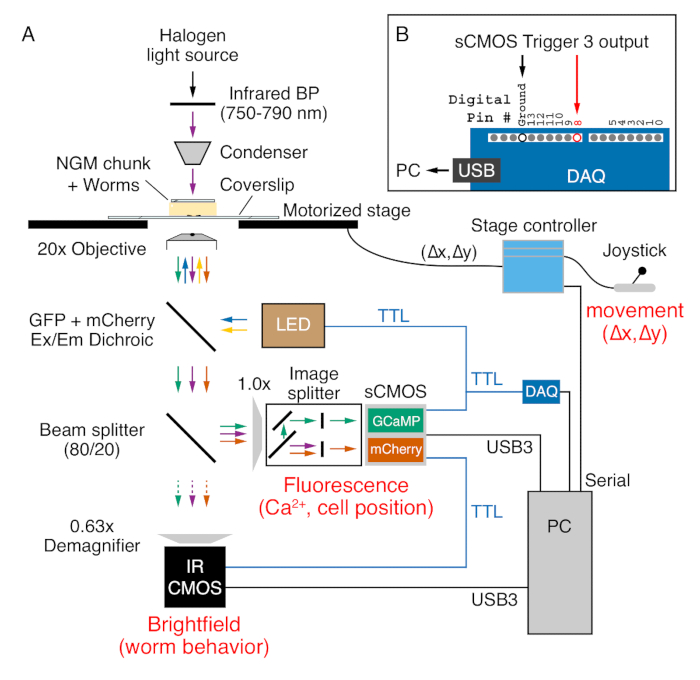
Figure 2. Widefield ratiometric Ca2+ imaging and behavior recording on an inverted epifluorescence microscope. (A) Worm position and behavior is captured in brightfield via a 20x (0.8 NA) Plan-Apochromat objective using infrared (750-790 nm) light (purple arrows) emitted from a halogen lamp through the NGM chunk. A joystick and motorized stage controller are used to maintain the worm in the field of view during recording. Stage position (Δx, Δy) is sent to the PC by the serial port. GCaMP5 and mCherry proteins expressed in the worm are excited using 470 nm (blue arrows) and 590 nm (yellow arrows) Light Emitting Diodes (LED). Emitted GCaMP5 (green arrows) and mCherry (orange arrows) fluorescence, along with the infrared light, passes through a multi-band dichroic mirror (see Table of Materials). An 80/20 beam-splitter sends 20% of the light through a 0.63x demagnifer for capture on an infrared-sensitive CMOS camera (purple arrow). The remaining 80% of the light is sent through the side-port of the microscope to an image splitter that separates the GCaMP5 and mCherry fluorescence onto separate halves of an sCMOS camera while removing infrared brightfield light. Data from both cameras are transferred to a PC via USB3 cables. The trigger ports from the fluorescence sCMOS camera (blue) are used to send +5V TTL triggers to the LED illumination system, the infrared brightfield CMOS camera, and the Digital Acquisition Device (DAQ). (B) Trigger 3 output TTL signals are detected by the DAQ at digital pin #8 and sent to the PC through a USB connection. These digital inputs trigger a 'where XY' serial command from a Bonsai software script (XY-stage-final), which reads the X and Y stage position for each GCaMP5/mCherry fluorescence/infrared image captured.
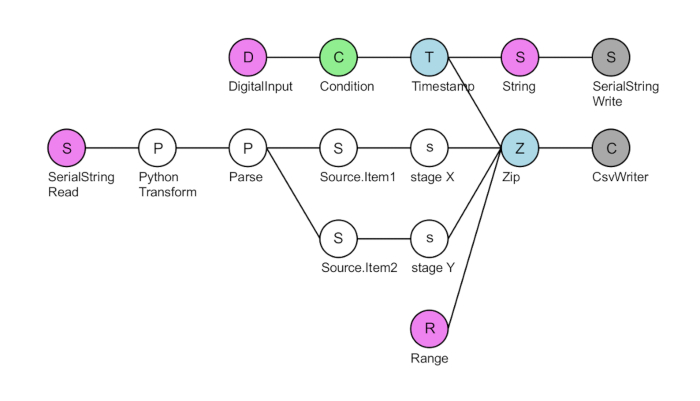
Figure 3: Layout of the Bonsai serial-stage communication script XY-stage-final. The top DigitalInput node (pink) reads TTL triggers coming into pin #8 of the DAQ. For each positive TTL voltage (green), the DAQ makes a timestamp (blue) and writes a 'Where XY?' string (pink) to the stage controller via the serial port (grey). The SerialStringRead node (pink) reads the X and Y coordinate response from the stage controller. This string is then converted into microns and separated into X and Y stage coordinates. Finally, these four streams are combined using a zip node (blue), and a four-column.csv file is written: a frame count of the TTL signals received (Range node, pink), the X and Y coordinates, and the interval between subsequent timepoints (typically ~ 50 ms when recording at 20 Hz).
Disclosures
The authors have nothing to disclose.
Materials
| C. elegans growth, cultivation, and mounting | |||
| Escherichia coli bacterial strain, OP50 | Caenorhabditis Genetic Center | OP50 | Food for C. elegans. Uracil auxotroph. E. coli B. Biosafety Level 1 |
| HSN GCaMP5+mCherry worm strain | Caenorhabditis Genetic Center | LX2004 | Integrated transgene using nlp-3 promoter to drive GCaMP5 and mCherry expression in HSN. Full genotype: vsIs183 [nlp-3p::GCaMP5::nlp-3 3'UTR + nlp-3p::mCherry::nlp-3 3'UTR + lin-15(+)], lite-1(ce314), lin-15(n765ts) X |
| lite-1(ce314), lin-15(n765ts) mutant strain for transgene preparation | author | LX1832 | Strain for recovery of high-copy transgenes after microinjection with pL15EK lin-15(n765ts) rescue plasmid. Also bears the linked lite-1(ce314) mutation which reduces blue-light sensitivity. Available from author by request |
| pL15EK lin-15a/b genomic rescue plasmid | author | pL15EK | Rescue plasmid for recovery of transgenic animals after injection into LX1832 lite-1(ce314), lin-15(n765ts) X strain. Available from author by request |
| pKMC299 plasmid | author | pKMC299 | Plasmid for expression of mCherry in the HSNs from the nlp-3 promoter. Has nlp-3 3' untranslated region |
| pKMC300 plasmid | author | pKMC300 | Plasmid for expression of GCaMP5 in the HSNs from the nlp-3 promoter. Has nlp-3 3' untranslated region |
| Potassium Phosphate Monobasic | Sigma | P8281 | For preparation of NGM plates |
| Potassium Phosphate Dibasic | Sigma | P5655 | For preparation of NGM plates |
| Magnesium Sulfate Heptahydrate | Amresco | 662 | For preparation of NGM plates |
| Calcium Chloride Dihydrate | Alfa Aesar | 12312 | For preparation of NGM plates |
| Peptone | Becton Dickinson | 211820 | For preparation of NGM plates |
| Sodium Chloride | Amresco | 241 | For preparation of NGM plates |
| Cholesterol | Alfa Aesar | A11470 | For preparation of NGM plates |
| Agar, Bacteriological Type A, Ultrapure | Affymetrix | 10906 | For preparation of NGM plates |
| 60 mm Petri dishes | VWR | 25384-164 | For preparation of NGM plates |
| 24 x 60 mm micro cover glasses, #1.5 | VWR | 48393-251 | Cover glass through which worms are imaged |
| 22 x 22 mm micro cover glasses, #1 | VWR | 48366-067 | Cover glass that covers the top of the agar chunk |
| Stereomicroscope with transmitted light base | Leica | M50 | Dissecting microscope for worm strain maintenance, staging, and mounting |
| Platinum iridium wire, (80:20), 0.2mm | ALFA AESAR | AA39526-BW | For worm transfer |
| Calcium imaging microscope | |||
| Anti-vibration air table | TMC | 63-544 | Micro-g' Lab Table 30" x 48" anti-vibration table with 4" CleanTop M6 on 25mm top |
| Inverted compound microscope | Zeiss | 431007-9902-000 | Axio Observer.Z1 inverted microscope |
| Sideport L80/R100 (3 position) | Zeiss | 425165-0000-000 | To divert 20% of output to brightfield (CMOS) camera, 80% to fluorescence (sCMOS) camera |
| Tilt Back Illumination Carrier | Zeiss | 423920-0000-000 | For infrared/behavior imaging |
| Lamphousing 12V/100W w/ Collector | Zeiss | 423000-9901-000 | For infrared/behavior imaging |
| Halogen lamp 12V/100W | Zeiss | 380059-1660-000 | For infrared/behavior imaging. White-light LEDs do not emit significant infrared light, so they will not allow brightfield imaging after the infrared bandpass filter |
| 32 mm Infrared bandpass filter (750-790 nm) for Halogen lamp | Zeiss | 447958-9000-000 | BP 750-790; DMR 32mm, for infrared illumination for brightfield and behavior |
| 6-filter Condenser Turret (LD 0.55 H/DIC/Ph), Motorized | Zeiss | 424244-0000-000 | For infrared/behavior imaging |
| Condenser & Shutter | Zeiss | 423921-0000-000 | For infrared/behavior imaging |
| Binocular eyepiece with phototube for infrared CMOS camera | Zeiss | 425536-0000-000 | For infrared/behavior imaging |
| Eyepiece 10x, 23mm | Zeiss | 444036-9000-000 | For worm localization on the agar chunk |
| C-Mount Adapter 2/3" 0.63x demagnifier | Zeiss | 426113-0000-000 | Mount for infrared CMOS camera |
| CMOS camera for infrared brightfield and behavior (1" sensor) | FLIR (formerly Point Grey Research) | GS3-U3-41C6NIR-C | Camera for brightfield imaging |
| USB 3.0 Host Controller Card | FLIR (formerly Point Grey Research) | ACC-01-1202 | Fresco FL1100, 4 Ports |
| 8 pins, 1m GPIO Cable, Hirose HR25 Circular Connector | FLIR (formerly Point Grey Research) | ACC-01-3000 | Cable for TTL triggering. The green wire connects to GPIO3 / Pin 4 and the brown wire connects to Ground / Pin 5 |
| Plan-Apochromat 20x/0.8 WD=0.55 M27 | Zeiss | 420650-9901-000 | Best combination of magnification, numerical aperture, and working distance |
| 6-cube Reflector Turret, Motorized | Zeiss | 424947-0000-000 | For fluorescence imaging |
| Fluorescence Light Train, Motorized | Zeiss | 423607-0000-000 | For fluorescence imaging |
| Fluorescence Shutter | Zeiss | 423625-0000-000 | For fluorescence imaging |
| GFP and mCherry dual excitation and emission filter cube (for microscope) | Zeiss | 489062-9901-000 | FL Filter Set 62 HE BFP+GFP+HcRed for fluorescence imaging |
| LED illumination system | Zeiss | 423052-9501-000 | Triggerable Colibri.2 LED system for fluorescent illumination |
| GFP LED module (470 nm) | Zeiss | 423052-9052-000 | Colibri.2 LED for GFP fluorescence excitation |
| mCherry LED module (590 nm) | Zeiss | 423052-9082-000 | Colibri.2 LED for mCherry fluorescence excitation |
| Iris stop slider for incident-light equipment | Zeiss | 000000-1062-360 | Field aperture iris to limit LED illumination to the camera field of view |
| C-Mount Adapter 1" 1.0x | Zeiss | 426114-0000-000 | Adapter for image-splitter and sCMOS fluorescence camera |
| Image splitter | Hamamatsu | A12801-01 | Gemini W-View, other image splitters may be used, but they may not be optimized for the large sensor size of the sCMOS cameras |
| GFP / mCherry dichroic mirror (image splitter) | Semrock | Di02-R594-25×36 | Splitting GCaMP5 from mCherry and infrared signals |
| GFP emission filter (image splitter) | Semrock | FF01-525/30-25 | Capturing GCaMP5 fluorescence |
| mCherry/ emission filter (image splitter) | Semrock | FF01-647/57-25 | This filter is necessary to exclude the infrared light used for brightfield imaging |
| sCMOS camera for fluorescence (1" sensor) | Hamamatsu | A12802-01 / C11440-22CU | Orca FLASH 4.0 V2. Newer models allow for separate image acquisition settings on separate halves of the sensor, allowing acquisition of two-channel images in combination with an image splitter |
| Motorized XY Stage | Märzhäuser | SCAN IM 130 x 100 | Stage movement; the XY resolution of this stage is 0.2µm per step |
| XY Stage controller with joystick | LUDL | MAC6000, XY joystick | Manual tracking of worms. MAC6000 controller should be connected to the PC through the serial (RS-232) port configured to 115200 baud |
| Digital Acquisition board (DAQ) | Arduino | Uno | Receiving TTL triggers from sCMOS camera. The Uno should be loaded with the standard Firmata package, and the computer USB port configured to 57600 baud |
| BNC Male to BNC Male Cable – 6 ft | Hosa Technology | HOBB6 | BNC connectors for TTL triggering |
| Gold-Plated BNC Male to SMA male coaxial cable (8.8") | uxcell | 6.08642E+11 | To connect the fluorescence camera trigger outputs |
| BNC turn head adapter | Hantek | RRBNCTH21 | BNC to Banana Plug Adapter (4mm) |
| BNC female to female connector | Diageng | 20130530009 | Female to female BNC adapter to connect the BNC output from the camera to the Banana Plug |
| Solderless flexible breadboard jumper wires | Z&T | GK1212827 | To connect the BNC trigger outputs to the Arduiono DAQ. Male to male. |
| High performace workstation | HP | Z820 | Windows 7, 64GB RAM, Dual Xeon processor, solid state C: drive, serial (RS-232) port, multiple PCIe3 slots for ethernet connectivity, USB 3.0 cards, and additional solid state drives |
| M.2 Solid state drive | Samsung | MZ-V5P512BW | High-speed streaming and analysis of image data |
| M.2 Solid state drive adapter for workstations | Lycom | DT-120 | M.2 to PCIe 3.0 4-lane adapter |
| Network attached storage | Synology | DS-2415+ | Imaging data storage and analysis |
| Hard disk drives | Western Digital | WD80EFZX | RED 8 TB, 5400 RPM Class SATA 6 Gb/s 128MB Cache 3.5 Inch. Storage of imaging data (10 drives + 2 drive redundancy) |
| Software | |||
| Fluorescence Acquisition | Hamamatsu | HCImage DIA | Recording of two channel (GCaMP5 and mCherry) fluorescence image sequences at 20 fps |
| Brightfield Acquisition | FLIR (formerly Point Grey Research) | Flycapture | Recording of brightfield JPEG image sequences |
| Stage Serial Port Reader | Bonsai | https://bitbucket.org/horizongir/bonsai | Facilitates tracking of worms during behavior |
| LED controller software | Zeiss | Micro Toolbox Test 2011 | To set up the intensity and trigger inputs for the different LEDs in the Colibri.2 unit |
| ImageJ | NIH | https://imagej.net/Fiji/Downloads | Simple review of image sequences and formatting changes for import into Ratiometric Quantitation software |
| Excel | Microsoft | 2002984-001-000001 | For generating subsets of comma-separated value data from Volocity for MATLAB analysis |
| Peak Finding | MATLAB | R2017a | Script used for Ratio peak feature calculations |
| Ratiometric Quantitation | Perkin Elmer | Volocity 6.3 | Facilitates calculation of ratiometric image channels, image segmentation for object finding, and ratio measurement of found objects |
| Scripts | |||
| AnalyzeGCaMP_2017.m | MATLAB | Mean Ratio and XY centroid script | Analyzes a ratiometric output in .csv format, consolidating objects and centroid positions, calculating ratio changes (ΔR/R), identifying peaks and peak features, and writing plots (with and without annotated peaks) in postscript format. For matrix file import, the script will output data into three folders: analyzed, peaks, and traces. 'Analyzed' output is a matrix file of the consolidated objects with six columns (units): time (s), area (square microns), ratio, X centroid (microns), Y centroid (microns), and ΔR/R. 'Peaks' output is a matrix file of the timepoints of found peaks, the amplitude (in ΔR/R), the peak width (s), and the prominance of the peak. 'Traces' output are postscript files of calcium traces. |
| XY-stage-final.bonsai | Bonsai | TTL-triggered DAQ and stage position serial port reader | Records X and Y stage position (in microns) when the attached Arduino receives a positive TTL signal from sCMOS camera during frame exposure. Script writes a .csv file with four columns: frame number, X position (microns), Y position (microns), and the time elapsed between frames (typically ~50 msec when recording at 20 fps). X and Y stage position from this output (columns 2 and 3, respectively) are added to the X and Y centroid positions from the AnalyzeGCaM |

