Creating a Winogradsky Column: A Method to Enrich the Microbial Species in a Sediment Sample
120,556 Views
•
•
Overview
Source: Elizabeth Suter1, Christopher Corbo1, Jonathan Blaize1
1 Department of Biological Sciences, Wagner College, 1 Campus Road, Staten Island NY, 10301
The Winogradsky column is a miniature, enclosed ecosystem used for enriching sediment microbial communities, especially those involved in sulfur cycling. The column was first used by Sergei Winogradsky in the 1880s and has since been applied in the study of many diverse microorganisms involved in biogeochemistry, such as photosynthesizers, sulfur oxidizers, sulfate reducers, methanogens, iron oxidizers, nitrogen cyclers, and more (1,2).
The majority of microorganisms on Earth are considered unculturable, meaning that they cannot be isolated in a test tube or on a petri dish (3). This is due to many factors, including that microorganisms depend on others for certain metabolic products. The conditions in a Winogradsky column closely mimic a microorganism's natural habitat, including their interactions with other organisms, and allows for them to be grown in a lab. Therefor, this technique permits scientists to study these organisms and understand how they are important to Earth's biogeochemical cycles without having to grow them in isolation.
Earth's environments are full of microorganisms which thrive in all types of habitats, such as soils, ocean water, clouds, and deep-sea sediments. In all habitats, microorganisms depend on each other. As a microorganism grows, it consumes particular substrates, including carbon-rich fuels like sugars as well as nutrients, vitamins, and respiratory gases like oxygen. When these important resources run out, different microorganisms with different metabolic needs can then bloom and thrive. For example, in the Winogradsky column, microbes first consume the added organic material while depleting the oxygen in the bottom layers of the column. Once the oxygen is used up, anaerobic organisms can then take over and consume different organic materials. This consecutive development of different microbial communities over time is called succession (4). Microbial succession is important in a Winogradsky column, where microbial activity changes the chemistry of the sediment, which then affects activity of other microbes and so on. Many microorganisms in soils and sediments also live along gradients, which are transitional zones between two different types of habitats based on the concentrations of substrates (5). At the correct spot in the gradient, a microbe can receive optimal amounts of different substrates. As a Winogradsky column develops, it begins to mimic these natural gradients, particularly in oxygen and sulfide (Fig. 1).
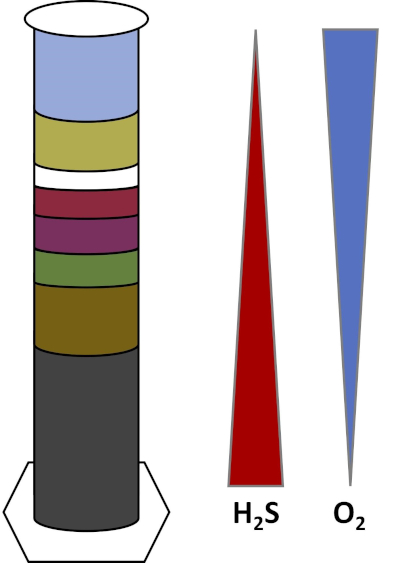
Figure 1: A representation of the oxygen (O2) and sulfide (H2S) gradients that develop in a Winogradsky column.
In a Winogradsky column, mud and water from a pond or wetland are mixed in a transparent column and allowed to incubate, typically in the light. Additional substrates are added to the column to give the community sources of carbon, usually in the form of cellulose, and sulfur. Photosynthesizers typically start to grow in the top layers of the sediment. These photosynthetic microorganisms are largely composed of cyanobacteria, which produce oxygen and appear as a green or red-brown layer (Fig. 2, Table 1). While photosynthesis produces oxygen, oxygen is not very soluble in water and it decreases below this layer (Fig. 1). This creates a gradient of oxygen, ranging from high concentrations of oxygen in the top layers to zero oxygen in the bottom layers. The oxygenated layer is called the aerobic layer and the layer without oxygen is called the anaerobic layer.
In the anaerobic layer, many different microbial communities can proliferate depending on the type and amount of substrates that are available, the source of the initial microbes, and the porosity of the sediment. At the bottom of the column, organisms that anaerobically break down organic matter can thrive. Microbial fermentation produces organic acids from the breakdown of cellulose. These organic acids can then be used by sulfate reducers, which oxidize those organics using sulfate, and produce sulfide as a byproduct. The activity of sulfate reducers is indicated if the sediment turns black, because iron and sulfide react to form black iron-sulfide minerals (Fig. 2, Table 1). The sulfide also diffuses upward, creating another gradient in which sulfide concentrations are high in the bottom of the column and low in the top of the column (Fig. 1).
Near the middle of the column, sulfur oxidizers take advantage of the supply of oxygen from above and sulfide from below. With the right amount of light, photosynthetic sulfur oxidizers can develop in these layers. These organisms are known as green and purple sulfur bacteria, and often appear as green, purple, or purple-red filaments and blotches (Fig. 2, Table 1). Green sulfur bacteria have a higher tolerance for sulfide and usually develop in the layer directly below purple sulfur bacteria. Above the purple sulfur bacteria, purple nonsulfur bacteria may also develop. These organisms photosynthesize using organic acids as electron donors instead of sulfide and often appear as a red, purple, orange, or brown layer. Nonphotosynthetic sulfur oxidizers can develop above the purple nonsulfur bacteria, and these usually appear as white filaments (Fig. 2, Table 1). Additionally, bubbles may also form in the Winogradsky column. Bubbles in the aerobic layers indicates the production of oxygen by the cyanobacteria. Bubbles in the anaerobic layers are likely due to the activity of methanogens, organisms which anaerobically break down organic matter and form methane as a byproduct.
| Position in Column | Functional group | Organism Examples | Visual Indicator |
| Top | Photosynthesizers | Cyanobacteria | Green or reddish-brown layer. Sometimes bubbles of oxygen. |
| Nonphotosynthetic sulfur oxidizers | Beggiatoa, Thiobacilus | White layer. | |
| Purple nonsulfur bacteria | Rhodomicrobium, Rhodospirilum, Rhodopseuodmonas | Red, purple, orange, or brown layer. | |
| Purple sulfur bacteria | Chromatium | Purple, or purple-red layer. | |
| Green sulfur bacteria | Chlorobium | Green layer. | |
| Sulfate Reducing Bacteria | Desulfovibrio, Desulfotomaculum, Desulfobacter, Desulfuromonas | Black layer. | |
| Bottom | Methanogens | Methanococcus, Methanosarcina | Sometimes bubbles of methane. |
Table 1: The main groups of bacteria that may appear in a classical Winogradsky column, from top to bottom. Examples of organisms from each group are given, and the visual indicators of each layer of organisms are listed. Based on Perry et al. (2002) and Rogan et al. (2005).
Procedure
1. Set-up
- To set up a Winogradsky column, you will need some basic supplies:
- A shovel, bucket, and bottle to collect the samples in the field
- A vertical, transparent vessel, such as a graduated cylinder or plastic water bottle of about 1L
- Plastic wrap and rubber bands
- large mixing bowls and large spoon to stir
- A sulfur source (egg yolk or calcium sulfate)
- A source of organic carbon (cellulose, in the form of shredded newspaper)
- A source of light (sunny window or desk lamp)
- Soil or mud collected from a marsh, wetland, pond, or stream
- Water from the same habitat
- OPTIONAL The following will be necessary for some of the optional experiments described in this protocol:
- Table salt
- Differently colored cellophane
- A source of iron (such as a nail or steel wool)
- A refrigerator with a light source
- A radiator near a light source
- If using a plastic water bottle, cut off the neck area so that the column is cylindrically shaped. Remove any wrappers so that light can penetrate through the plastic.
- Raw eggs may contain Salmonella and should be handled with care. Proper hand-washing techniques should be followed. Alternatively, boiled egg can be used. Additionally, there is no way to know for sure if mud or sediment is contaminated with sewage or other harmful substance. Gloves should be used when mixing the mud and setting up the column.
2. Assembling a Winogradsky Column
- Using the shovel, dig up and collect mud into the bucket. The sediments should be near the water's edge and completely saturated with water. You will need enough mud to fill each Winogradsky column. Collect some water from the same source into the sample bottle (approximately 3000 mL per column is needed).
- In the lab, transfer enough mud to the first mixing bowl to fill ~75% of your 1-liter volume column. Next, sift through to remove large rocks, twigs, or leaves while using the spoon to break apart clumps.
- Add some of the water that you collected to the mixing bowl while stirring. Add until the consistency of the water-mud mixture is like a milkshake. Continue to make sure there are no clumps.
- Transfer about ⅓ of the water-mud milkshake to the second bowl. Add the egg yolk and a handful of shredded newspaper and mix.
- Add the mixture of mud, egg yolk, and newspaper to the column until the column is about ¼ full.
- Add the regular water-mud mixture into the column until the column is about ¾ full.
- Add the additional water to the column, leaving only a small space (~½ inch) of air on top.
- Cover the column with plastic wrap and secure with a rubber band.
- Incubate the column in the light at room temperature.
- For the next 4 to 8 weeks, monitor changes in the Winogradsky column for the development of different colored layers and the formation of bubbles, as described in Table 1. Additionally, you should record the time it takes for different layers to develop.
3. Optional Modifications to the Classical Winogradsky Column
- Add 25-50g of salt per 1L Winogradsky column to the collected mud before adding water and stirring (step 2.3). The addition of salt selects for halophilic (salt-loving) bacteria.
- Alternate substrates, such as iron, in the form of a nail or steel wool, can be added to the column along with the egg yolk and the shredded newspaper (step 2.4). This will enrich iron-oxidizing bacteria, such as Gallionella, and will appear as a rust-colored layer.
- Instead of room temperature (step 2.9), the column can be incubated near a radiator to select for thermophilic (heat-loving) bacteria or in a refrigerator with a light source to select for psychrophilic (cold-loving) bacteria.
- The amount of light a column receives as it incubates (step 2.9) can also be varied by placing different columns in high light, low light, or darkness.
- The wavelength of incoming light can be limited by covering the column with differently shaded cellophanes as it incubates (step 2.9) to determine which colors select for different bacterial groups.
4. Data Analysis
- After 1-3 weeks, some green coloring on the top of the mud layer of the classical Winogradsky column should be visible (Fig. 2A). These are the first signs of growth of the cyanobacterial layer.
- Over time, continue to monitor the appearance and evolution of the different layers, each indicative of the different bacterial types. HINT: Refer to the Concepts and Table 1 to understand which bacteria contribute to the different layers.

Figure 2A: A photo of a classical Winogradsky column that has incubated at room temperature for 21 days. Note the green sediment, indicative of cyanobacteria, in the upper portion of the column.
- If modifications to the classical Winogradsky column were also prepared, compare the results of each column.
- Observe the layers in each of the modified Winogradsky columns. Take note of the following:
- Do the columns exhibit the same number of layers?
- Are the layers the same color and thickness?
- Do the layers occur at the same depths?
- How long did each column take to develop?
- Did one column develop more slowly than the others?
- Observe the layers in each of the modified Winogradsky columns. Take note of the following:
5. Results
- In this experiment, water and sediment were collected from a freshwater habitat. Two Winogradsky columns were constructed and allowed to develop: a classical Winogradsky column incubated in the light at room temperature (Fig. 2A) and a Winogradsky column incubated in the dark at room temperature (Fig. 2B).

Figure 2B: A photo of classical Winogradsky column (left), incubated at room temperature in light for 68 days and a Winogradsky column incubated at room temperature in the dark for 68 days (right).
- After allowing the columns to develop for 7-9 weeks, the layers in the classical column can be compared to the column incubated in the dark (Fig. 2B). In the classical Winogradsky column, a green cyanobacterial layer can be observed near the top of the tube. Near the center of the tube, a red-purple layer can be observed, indicative of purple nonsulfur bacteria. Under this layer, a purple-red layer is observed, indicative of purple sulfur bacteria. Directly under this layer, black sediment can be observed in the anaerobic region of the column, indicative of sulfate reducing bacteria.
- The column grown in the dark (Fig. 2B) developed differently than the classical Winogradsky column. Like the classical column, the dark column yielded black sediment near the bottom of the column, indicative of sulfate reducing bacteria. The dark column did not yield the green cyanobacterial layer, nor the red, purple, or green layers indicative of purple nonsulfur, purple sulfur, and green sulfur bacteria, respectively. These groups are dependent on light for growth, and therefore unable to grow in the dark.
- The precise results of each Winogradsky column will vary widely with their incubation conditions and their source habitats.
- Microbial communities originating from freshwater habitats will not be accustomed to high salt concentrations and the addition of salt may slow down or inhibit growth. Conversely, there may be sufficient halophilic bacteria in brackish and saltwater habitats so that the addition of salts makes no difference or even enhances the growth of particular layers when compared to a column without added salts.
- Sandy sediments are more porous than muddy sediments. If enough sulfide is produced in such porous sediments, sulfides can diffuse all the way to the top of the column and inhibit growth of aerobic organisms. In this case, the column may only contain layers indicative of anaerobes and may not contain any aerobes, such as the cyanobacteria.
- Freshwater generally contains less sulfate than saltwater. Sulfate is important for the growth of sulfate-reducing bacteria. Sulfate reducers create sulfide as a byproduct and are indicated by the development of a black layer in the bottom of the column. If sulfate is not supplemented to freshwater communities, sulfate reducers may not produce enough sulfide. The creation of the sulfide byproduct is important for the growth of green and purple sulfur bacteria and the nonphotosynthetic sulfur oxidizers. In these cases, sulfur oxidizers can still grow using the egg yolk as a source of sulfur, even if the sulfate reducers (black layer) never develop.
- Different wavelengths of light should select for organisms with different absorption pigments. A column kept in the dark will only allow for nonphotosynthetic organisms to grow, including sulfate reducers, iron oxidizers, and methanogens. Photosynthesizers have pigments that absorb light at different wavelengths within the visible range (~400-700nm). By covering a column with, for example, blue cellophane, blue light (~450-490nm) is blocked from entering the column. All of the photosynthesizers in the column have pigments which require the blue wavelengths (6) and their growth should be inhibited. On the other hand, red cellophane will block light of ~635-700nm. These wavelengths are important for the pigments used by cyanobacteria (6), while purple sulfur, green sulfur, and purple nonsulfur bacteria may still be able to grow.
- Different microbial communities may have vastly different adaptive abilities to cope with changes in temperatures. High temperatures can enhance rates of microbial activity when sufficient thermophiles are present. On the other hand, in the absence of thermophiles, high temperatures may decrease overall microbial activity. Similarly, low temperatures may decrease overall microbial activity unless the microbial community contains sufficient psychrophiles.
Most of the Earth's microorganisms cannot be cultured in a lab, often because they rely on other microbes within their native communities. A Winogradsky column, named for its inventor Sergei Winogradsky, is a miniature, enclosed ecosystem which enriches the microbial communities within a sediment sample, enabling scientists to study many of the microbes that play a vital role in Earth's biogeochemical processes, without needing to isolate and culture them individually.
Typically, mud and water from an ecosystem, such as a pond or a marsh, are mixed. As an optional experiment, salt can be added to this mixture to enrich various halophile species. Next, a small portion of the mixture is supplemented with carbon, usually in the form of cellulose from newspaper, and sulfur, usually from an egg yolk. For another optional experiment, a nail can be added to this mixture to enrich certain Gallionella species. This new mixture is then added to a transparent column, so that the column is one quarter full. Finally, the rest of the mud mixture and more water is added to the column until it is most of the way full.
Succession, which refers to the consecutive development of different microbial communities over time, can be observed in real time with a Winogradsky column. As microbes grow within the column, they consume specific substrates and change the chemistry of their environment. When their substrates are depleted, the original microbes die off and microbes with different metabolic needs can flourish in the altered environment. Over time, visibly distinct layers begin to form, each containing parts of a bacterial community with different microenvironmental needs.
For example, photosynthetic microbes, largely composed of cyanobacteria, form green or red-brown layers near the top of the column. Since photosynthesis produces oxygen, often seen as bubbles in the top portion of the column, a gradient is formed with the highest oxygen concentrations near the top, and the lowest towards the bottom. Depending upon the available substrates, different microbial communities can grow in the anaerobic bottom layer. Bubbles in this layer can indicate the presence of methanogens, which create methane gas via fermentation. Here, the microbial fermentation of cellulose results in organic acids. Sulfate reducers oxidize those acids to produce sulfide, and their activity is indicated by black sediment. Sulfide diffuses upward in the column, creating yet another gradient where sulfide concentrations are highest towards the bottom of the column, and lowest near the top. Towards the middle of the column, sulfur oxidizers utilize the oxygen from above and sulfide from below. With adequate light, photosynthetic sulfur oxidizers, such as green and purple sulfur bacteria, develop. Green sulfur bacteria tolerate higher sulfide concentrations. Thus, they grow directly below the purple sulfur bacteria. Directly above this layer, purple non-sulfur bacteria form a red-orange layer. Nonphotosynthetic sulfur oxidizers are indicated by the presence of white filaments.
Conditions such as light and temperature can also be varied to enrich other communities. In this video, you will learn how to construct a Winogradsky column, and vary the growing conditions and substrates to enrich specific microbial communities.
First, locate an appropriate aquatic ecosystem, such as a pond or marsh. The sediment samples should come from the area near the water's edge, and be completely saturated with water. Then, use a shovel and a bucket to collect one to two liters of the saturated mud. Next, obtain approximately three liters of fresh water from the same source and return to the lab with the field samples.
In the lab, put on the appropriate personal protective equipment, including a lab coat and gloves. Now, transfer approximately 750 milliliters of mud to a mixing bowl. Then, sift through the mud to remove large rocks, twigs, or leaves and use a spoon to break apart any clumps. Next, add some of the fresh water to the mixing bowl, and stir with a large spoon. Add water until the consistency of the water-mud mixture is similar to a milkshake. Continue to make sure there are no clumps.
As an optional experiment, select for halophilic bacteria by adding 25 to 50 milligrams of salt to the mud mixture.
Then, transfer approximately 1/3 of the water-mud mixture to a second mixing bowl. Add one egg yolk and a handful of shredded newspaper to the bowl. Next, add this mixture to the column, until it is about 1/4 full. Next, add the water-mud mixture without the egg and newspaper to the column, until it is approximately 3/4 full. Then, add more water to the column, leaving a 1/2 inch space on top. Cover the column with plastic wrap and secure it with a rubber band.
Incubate the column in the light near a window at room temperature for the next four to eight weeks. Throughout the incubation period, monitor changes in the Winogradsky column at least once a week for the development of different colored layers and the formation of bubbles. Additionally, record the time it takes for different layers to develop.
Another modification that can be done is incubating the column near a radiator to select for thermophilic bacteria, or in a refrigerator to select for psychrophilic bacteria. Vary the light conditions by placing different columns in high light, low light, or darkness to incubate. Alternatively, limit the wavelength of incoming light by covering the column with different shades of cellophane to determine which colors select for different bacterial groups. For another optional experiment, to enrich iron-oxidizing bacteria, add a nail to the mud-water mixture prior to the addition of newspaper and an egg yolk.
After one to two weeks, growth of the cyanobacterial layer is indicated by a green or red-brown film on top of the mud layer of the classical Winogradsky column. Over time, the appearance and evolution of the different layers is monitored, each indicative of the different types of bacteria present. When comparing a column grown in the dark to a traditional Winogradsky column, we see the dark treatment yields the black layer at the bottom of the column, indicative of sulfate-reducing bacteria.
The dark column may also yield other layers, depending on other incubation conditions. Additionally, the dark column doesn't yield the green cyanobacterial layer, nor the red, purple, or green layers indicative of purple non-sulfur, purple sulfur, and green sulfur bacteria respectively. These groups are dependent on light for growth.
Results
In this experiment, water and sediment were collected from a freshwater habitat. Two Winogradsky columns were constructed and allowed to develop: a classical Winogradsky column incubated in the light at room temperature (Fig. 2A) and a Winogradsky column incubated in the dark at room temperature (Fig. 2B).

Figure 2B: A photo of classical Winogradsky column (left), incubated at room temperature in light for 68 days and a Winogradsky column incubated at room temperature in the dark for 68 days (right).
After allowing the columns to develop for 7-9 weeks, the layers in the classical column can be compared to the column incubated in the dark (Fig. 2B). In the classical Winogradsky column, a green cyanobacterial layer can be observed near the top of the tube. Near the center of the tube, a red-purple layer can be observed, indicative of purple nonsulfur bacteria. Under this layer, a purple-red layer is observed, indicative of purple sulfur bacteria. Directly under this layer, black sediment can be observed in the anaerobic region of the column, indicative of sulfate reducing bacteria.
The column grown in the dark (Fig. 2B) developed differently than the classical Winogradsky column. Like the classical column, the dark column yielded black sediment near the bottom of the column, indicative of sulfate reducing bacteria. The dark column did not yield the green cyanobacterial layer, nor the red, purple, or green layers indicative of purple nonsulfur, purple sulfur, and green sulfur bacteria, respectively. These groups are dependent on light for growth, and therefore unable to grow in the dark.
The precise results of each Winogradsky column will vary widely with their incubation conditions and their source habitats.
Microbial communities originating from freshwater habitats will not be accustomed to high salt concentrations and the addition of salt may slow down or inhibit growth. Conversely, there may be sufficient halophilic bacteria in brackish and saltwater habitats so that the addition of salts makes no difference or even enhances the growth of particular layers when compared to a column without added salts.
Sandy sediments are more porous than muddy sediments. If enough sulfide is produced in such porous sediments, sulfides can diffuse all the way to the top of the column and inhibit growth of aerobic organisms. In this case, the column may only contain layers indicative of anaerobes and may not contain any aerobes, such as the cyanobacteria.
Freshwater generally contains less sulfate than saltwater. Sulfate is important for the growth of sulfate-reducing bacteria. Sulfate reducers create sulfide as a byproduct and are indicated by the development of a black layer in the bottom of the column. If sulfate is not supplemented to freshwater communities, sulfate reducers may not produce enough sulfide. The creation of the sulfide byproduct is important for the growth of green and purple sulfur bacteria and the nonphotosynthetic sulfur oxidizers. In these cases, sulfur oxidizers can still grow using the egg yolk as a source of sulfur, even if the sulfate reducers (black layer) never develop.
Different wavelengths of light should select for organisms with different absorption pigments. A column kept in the dark will only allow for nonphotosynthetic organisms to grow, including sulfate reducers, iron oxidizers, and methanogens. Photosynthesizers have pigments that absorb light at different wavelengths within the visible range (~400-700nm). By covering a column with, for example, blue cellophane, blue light (~450-490nm) is blocked from entering the column. All of the photosynthesizers in the column have pigments which require the blue wavelengths (6) and their growth should be inhibited. On the other hand, red cellophane will block light of ~635-700nm. These wavelengths are important for the pigments used by cyanobacteria (6), while purple sulfur, green sulfur, and purple nonsulfur bacteria may still be able to grow.
Different microbial communities may have vastly different adaptive abilities to cope with changes in temperatures. High temperatures can enhance rates of microbial activity when sufficient thermophiles are present. On the other hand, in the absence of thermophiles, high temperatures may decrease overall microbial activity. Similarly, low temperatures may decrease overall microbial activity unless the microbial community contains sufficient psychrophiles.
Applications and Summary
The Winogradsky column is an example of an interdependent microbial ecosystem. After mixing mud, water, and additional carbon and sulfur substrates in a vertical column, the stratified ecosystem should stabilize into separate, stable zones over several weeks. These zones are occupied by different microorganisms which flourish at a particular spot along the gradient between the sulfide-rich sediment in the bottom and the oxygen-rich sediment at the top. By manipulating the conditions and substrates within the Winogradsky column, the presence and activity of different microorganisms such as halophiles, thermophiles, psychrophiles, sulfur oxidizers, sulfur reducers, iron oxidizers, and photosynthesizers can be observed.
References
- Zavarzin G. (2006). Winogradsky and modern microbiology. Microbiology 75(6): 501-511. doi: 10.1134/s0026261706050018
- Esteban DJ, Hysa B, and Bartow-McKenney C (2015). Temporal and Spatial Distribution of the Microbial Community of Winogradsky Columns. PLoS ONE 10(8): e0134588. doi:10.1371/journal.pone.0134588
- Lloyd KG, Steen AD, Ladau J, Yin J, and Crosby L. (2018). Phylogenetically novel uncultured microbial cells dominate Earth microbiomes. mSystems 3(5): e00055-18. doi:10.1128/mSystems.00055-18
- Anderson DC, and Hairston RV (1999). The Winogradsky Column & Biofilms: Models for Teaching Nutrient Cycling & Succession in an Ecosystem. The American Biology Teacher, 61(6): 453-459. doi: 10.2307/4450728
- Dang H, Klotz MG, Lovell CR and Sievert SM (2019) Editorial: The Responses of Marine Microorganisms, Communities and Ecofunctions to Environmental Gradients. Frontiers in Microbiology 10(115). doi: 10.3389/fmicb.2019.00115
- Stomp M, Huisman J, Stal LJ, and Matthijs HCP. (2007) Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME Journal. 1(4): 271-282. doi:10.1038/ismej.2007.59
- Perry JJ, Staley JT, and Lory S. (2002) Microbial Life, First Edition, published by Sinauer Associates
- Rogan B, Lemke M, Levandowsky M, and Gorrel T. (2005) Exploring the Sulfur Nutrient Cycle Using the Winogradsky Column. The American Biology Teacher, 67(6): 348-356. doi: 10.2307/4451860
Transcript
Most of the Earth’s microorganisms cannot be cultured in a lab, often because they rely on other microbes within their native communities. A Winogradsky column, named for its inventor Sergei Winogradsky, is a miniature, enclosed ecosystem which enriches the microbial communities within a sediment sample, enabling scientists to study many of the microbes that play a vital role in Earth’s biogeochemical processes, without needing to isolate and culture them individually.
Typically, mud and water from an ecosystem, such as a pond or a marsh, are mixed. As an optional experiment, salt can be added to this mixture to enrich various halophile species. Next, a small portion of the mixture is supplemented with carbon, usually in the form of cellulose from newspaper, and sulfur, usually from an egg yolk. For another optional experiment, a nail can be added to this mixture to enrich certain Gallionella species. This new mixture is then added to a transparent column, so that the column is one quarter full. Finally, the rest of the mud mixture and more water is added to the column until it is most of the way full.
Succession, which refers to the consecutive development of different microbial communities over time, can be observed in real time with a Winogradsky column. As microbes grow within the column, they consume specific substrates and change the chemistry of their environment. When their substrates are depleted, the original microbes die off and microbes with different metabolic needs can flourish in the altered environment. Over time, visibly distinct layers begin to form, each containing parts of a bacterial community with different microenvironmental needs.
For example, photosynthetic microbes, largely composed of cyanobacteria, form green or red-brown layers near the top of the column. Since photosynthesis produces oxygen, often seen as bubbles in the top portion of the column, a gradient is formed with the highest oxygen concentrations near the top, and the lowest towards the bottom. Depending upon the available substrates, different microbial communities can grow in the anaerobic bottom layer. Bubbles in this layer can indicate the presence of methanogens, which create methane gas via fermentation. Here, the microbial fermentation of cellulose results in organic acids. Sulfate reducers oxidize those acids to produce sulfide, and their activity is indicated by black sediment. Sulfide diffuses upward in the column, creating yet another gradient where sulfide concentrations are highest towards the bottom of the column, and lowest near the top. Towards the middle of the column, sulfur oxidizers utilize the oxygen from above and sulfide from below. With adequate light, photosynthetic sulfur oxidizers, such as green and purple sulfur bacteria, develop. Green sulfur bacteria tolerate higher sulfide concentrations. Thus, they grow directly below the purple sulfur bacteria. Directly above this layer, purple non-sulfur bacteria form a red-orange layer. Nonphotosynthetic sulfur oxidizers are indicated by the presence of white filaments.
Conditions such as light and temperature can also be varied to enrich other communities. In this video, you will learn how to construct a Winogradsky column, and vary the growing conditions and substrates to enrich specific microbial communities.
First, locate an appropriate aquatic ecosystem, such as a pond or marsh. The sediment samples should come from the area near the water’s edge, and be completely saturated with water. Then, use a shovel and a bucket to collect one to two liters of the saturated mud. Next, obtain approximately three liters of fresh water from the same source and return to the lab with the field samples.
In the lab, put on the appropriate personal protective equipment, including a lab coat and gloves. Now, transfer approximately 750 milliliters of mud to a mixing bowl. Then, sift through the mud to remove large rocks, twigs, or leaves and use a spoon to break apart any clumps. Next, add some of the fresh water to the mixing bowl, and stir with a large spoon. Add water until the consistency of the water-mud mixture is similar to a milkshake. Continue to make sure there are no clumps.
As an optional experiment, select for halophilic bacteria by adding 25 to 50 milligrams of salt to the mud mixture.
Then, transfer approximately 1/3 of the water-mud mixture to a second mixing bowl. Add one egg yolk and a handful of shredded newspaper to the bowl. Next, add this mixture to the column, until it is about 1/4 full. Next, add the water-mud mixture without the egg and newspaper to the column, until it is approximately 3/4 full. Then, add more water to the column, leaving a 1/2 inch space on top. Cover the column with plastic wrap and secure it with a rubber band.
Incubate the column in the light near a window at room temperature for the next four to eight weeks. Throughout the incubation period, monitor changes in the Winogradsky column at least once a week for the development of different colored layers and the formation of bubbles. Additionally, record the time it takes for different layers to develop.
Another modification that can be done is incubating the column near a radiator to select for thermophilic bacteria, or in a refrigerator to select for psychrophilic bacteria. Vary the light conditions by placing different columns in high light, low light, or darkness to incubate. Alternatively, limit the wavelength of incoming light by covering the column with different shades of cellophane to determine which colors select for different bacterial groups. For another optional experiment, to enrich iron-oxidizing bacteria, add a nail to the mud-water mixture prior to the addition of newspaper and an egg yolk.
After one to two weeks, growth of the cyanobacterial layer is indicated by a green or red-brown film on top of the mud layer of the classical Winogradsky column. Over time, the appearance and evolution of the different layers is monitored, each indicative of the different types of bacteria present. When comparing a column grown in the dark to a traditional Winogradsky column, we see the dark treatment yields the black layer at the bottom of the column, indicative of sulfate-reducing bacteria.
The dark column may also yield other layers, depending on other incubation conditions. Additionally, the dark column doesn’t yield the green cyanobacterial layer, nor the red, purple, or green layers indicative of purple non-sulfur, purple sulfur, and green sulfur bacteria respectively. These groups are dependent on light for growth.
