Quadruply Metal-Metal Bonded Paddlewheels
14,722 Views
•
•
Genel Bakış
Source: Corey Burns, Tamara M. Powers, Department of Chemistry, Texas A&M University
Paddlewheel complexes are a class of compounds comprised of two metal ions (1st, 2nd, or 3rd row transition metals) held in proximity by four bridging ligands (most commonly formamidinates or carboxylates) (Figure 1). Varying the identity of the metal ion and the bridging ligand provides access to large families of paddlewheel complexes. The structure of paddlewheel complexes allows for metal-metal bonding, which plays a vital role in the structure and reactivity of these complexes. Due to the diversity of electronic structures that are available to paddlewheel complexes – and the corresponding differences in M-M bonding displayed by these structures – paddlewheel complexes have found application in diverse areas, such as in homogeneous catalysis and as building blocks for metal-organic frameworks (MOFs). Understanding the electronic structure of the M-M bonds in paddlewheel complexes is critical to understanding their structures and thus to application of these complexes in coordination chemistry and catalysis.
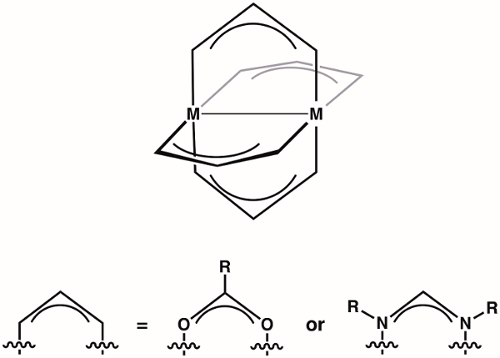
Figure 1. General structure of paddlewheel complexes, where M can be a 1st, 2nd, or 3rd row transition metal.
When two transition metals are held in close proximity the d-orbitals overlap, which can result in the formation of M-M bonds. Overlapping d-orbitals can form three types of bonds – σ, π, and δ – depending on the symmetry of the orbitals involved. If we assign the molecular z-axis to be coplanar with the M-M bond, a σ bond is formed by overlap of the dz2 orbitals and π bonds are formed by overlap of the dxz and dyz orbitals. δ bonds are generated by overlap of d-orbitals that have two planar nodes (dxy and dx2–y2). As a result, all four lobes of the d-orbital overlap and the corresponding δ bond has two planar nodes (Figure 2). In theory, with the addition of δ bonds, paddlewheel complexes are capable of supporting quintuple bonds, or five bonds between metal atoms.1 In most complexes, the dx2–y2 forms strong metal-ligand bonds and does not meaningfully contribute to M-M bonding. Thus, quadruple bonds are the maximum bond order in many complexes.
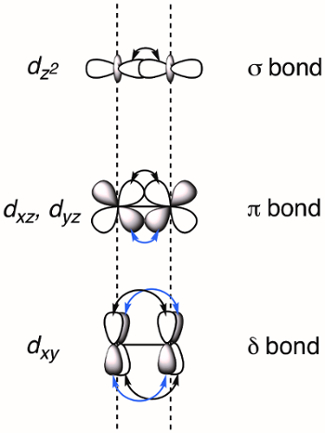
Figure 2. Visual representation of σ, π, and δ bonding MOs resulting from the linear combination of metal d-orbitals. The dz2 atomic orbitals have the best spatial overlap, followed by the dxz and dyz orbitals. The dxy atomic orbitals have the least amount of spatial overlap.
In this video, we will synthesize the dimolybdenum paddlewheel complex Mo2(ArNC(H)NAr)4, where Ar = p-(MeO)C6H4, which features a quadruple bond. We will characterize the compound by NMR spectroscopy and use X-ray crystallography to study the M-M bond.
İlkeler
We begin by constructing an MO diagram of the M-M bond within the general dimolybdenum complex Mo2(ArNC(H)NAr)4. First, we need to define our axes. Assuming the highest available symmetry, Mo2(ArNC(H)NAr)4 is in the point group D4h (Figure 3). The z-axis is by definition assigned to the axis with highest rotational symmetry (primary axis), which in this case is a C4 rotation axis that lies along the Mo-Mo bond. By convention, the x- and y-axis lie along the M-L bonds; in our specific case, this means that the x- and y-axes are collinear with the Mo-N vectors. According to our axis assignments, the dx2–y2 orbital on each Mo atom is involved in metal-ligand bonding. That leaves the dxy, dxz, dyz, and dz2 orbitals for M-M bonding.
The MO diagram that describes the M-M bond in Mo2(ArNC(H)NAr)4 is shown in Figure 4. Linear combination of the dz2 orbital on each M atom results in σ and σ* MOs. The dxz and dyz orbitals form π and π* MOs. Finally, linear combination of the dxy atomic orbitals gives rise to the δ and δ* MOs. The δ bond exhibits the least amount of spatial overlap between the atomic orbitals and, as a result, the relative energy of the bonding orbitals is σ < π < δ (Figure 2). This corresponds to bond strengths, where a σ bond is stronger than a π bond, which is stronger than a δ bond. We fill the corresponding MOs with the total number of d e– for both Mo center, which is 8 (Mo2+, d4). This leads to a bond order of 4, which is consistent with a quadruple bond.
In this video, we will use X-ray crystallography to observe the Mo-Mo bond length in the Mo2(ArNC(H)NAr)4 complex. With the Mo-Mo bond distance from solid state structure, we can find the formal shortness ratio (FSR), which is the normalized value of the M-M bond. The FSR is calculated for a bond A-B using Equation 1, which is simply the ratio of the bond distance observed in the solid state (DA-B) to the sum of the atomic radii (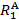 and
and 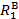 ) of the individual atoms.
) of the individual atoms.
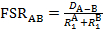 (1)
(1)
The FSR value is normalized for atomic radius and thus provides a fast and convenient way to compare M-M bond distances, not only between different metal types, but also to bond distances between non-metal atoms.
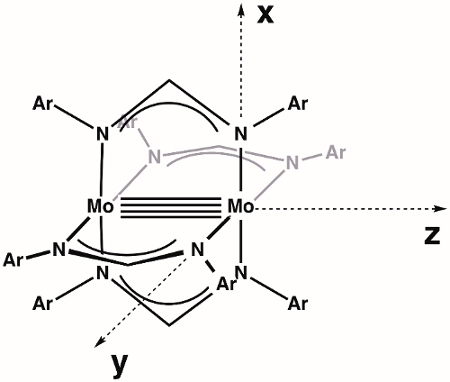
Figure 3. Defined axes for the molecule Mo2(ArNC(H)NAr)4, assuming highest symmetry (D4h).
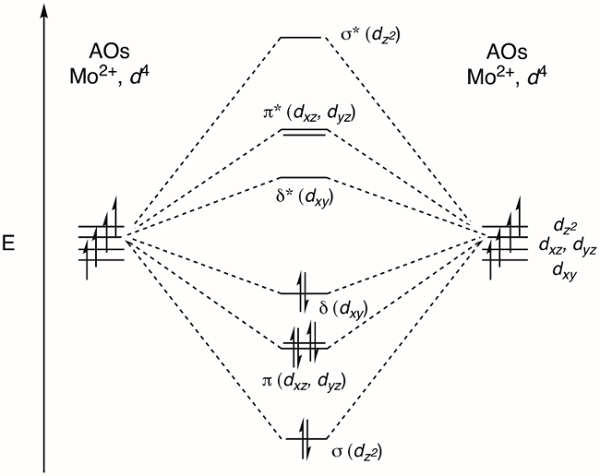
Figure 4. MO diagram of the M-M bonding in Mo2(ArNC(H)NAr)4.
Prosedür
1. Synthesis of Ligand ArN(H)C(H)NAr, Where Ar = p-(MeO)C6H4 (Figure 5)2
- Combine 6.0 g (0.050 mol) of p-anisidine and 4.2 mL (0.025 mol) of triethylorthoformate in a 100 mL round bottom flask with a magnetic stir bar.
- Attach a distillation head to the reaction flask.
- With stirring, heat the reaction in an oil bath to reflux (120 °C). Once reflux is achieved, the byproduct ethanol should begin to distill from the reaction. Collect the ethanol in a beaker placed at the end of the distillation head.
- Heat the reaction until the distillation of ethanol ceases (at least 1.5 h).
- Remove the flask from the oil bath and allow the reaction mixture to cool to room temperature. A precipitate should form. If the product does not precipitate, place the flask in an ice bath and scratch the bottom of the flask with a spatula to encourage crystallization.
- Recrystallize the product from a minimal amount of boiling toluene (for a more detailed procedure, please review the "Purifying Compounds by Recrystallization" video in the Essentials of Organic Chemistry series).
- Collect the product by filtration through a fritted funnel and wash with 10 mL of hexanes.
- Isolate the white product and allow it to dry in the air.
- Collect a 1H NMR of the solid using CDCl3.
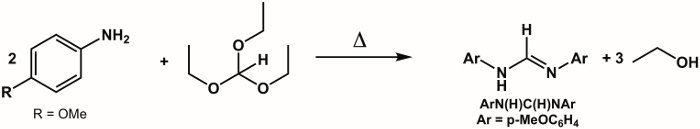
Figure 5. Synthesis of ArN(H)C(H)NAr, where Ar = p-MeOC6H4.
2. Setup of the Schlenk Line
NOTE: For a more detailed procedure, please review the "Schlenk Lines Transfer of Solvent" video in the Essentials of Organic Chemistry series. Schlenk line safety should be reviewed prior to conducting this experiment. Glassware should be inspected for star cracks before use. Care should be taken to ensure that O2 is not condensed in the Schlenk line trap if using liquid N2. At liquid N2 temperature, O2 condenses and is explosive in the presence of organic solvents. If it is suspected that O2 has been condensed or a blue liquid is observed in the cold trap, leave the trap cold under dynamic vacuum. Do NOT remove the liquid N2 trap or turn off the vacuum pump. Over time the liquid O2 will sublime into the pump; it is only safe to remove the liquid N2 trap once all of the O2 has sublimed.
- Close the pressure release valve.
- Turn on the N2 gas and the vacuum pump.
- As the Schlenk line vacuum reaches its minimum pressure, prepare the cold trap with either liquid N2 or dry ice/acetone.
- Assemble the cold trap.
3. Synthesis of Mo2(ArNC(H)NAr)4 (Figure 6)2
CAUTION: The molybdenum source used in the synthesis of Mo2(ArNC(H)NAr)4 is Mo(CO)6, which is highly toxic and may be fatal if inhaled, absorbed through the skin, or swallowed. CO is generated during the reaction. Therefore, the synthesis must be conducted in a well-ventilated hood.
- Use standard Schlenk line techniques for the synthesis of Mo2(ArNC(H)NAr)4 (see the "Synthesis of a Ti(III) Metallocene Using Schlenk line Technique" video).
- Add 0.34 g (1.3 mmol) Mo(CO)6 and 1.0 g (3.9 mmol) of ArN(H)C(H)NAr to a 100 mL Schlenk flask and prepare the Schlenk flask for the cannula transfer of solvent.
- Add 20 mL of degassed o-dichlorobenzene to the Schlenk flask via cannula transfer.
- Fit the Schlenk flask with a condenser connected to the N2 gas line.
- Reflux the reaction for 2 h (180 °C) in a silicone-oil bath.
NOTE: Mo(CO)6 is volatile and will condense on the sides of the Schlenk flask during the reaction. To obtain higher yields, periodically re-dissolve any sublimed Mo(CO)6 by pulling the flask out of the oil bath and gently swirling the solvent in the flask. - Remove the Schlenk flask from the oil bath and allow the mixture to cool to room temperature.
- Filter the brown solution through a fritted funnel and wash the yellow precipitate with 10 mL of hexanes, followed by 5 mL of reagent grade acetone. The Mo2(ArNC(H)NAr)4 does decompose slowly in solution when O2 is present. Therefore, the filtration should be done promptly once the reaction is removed from N2.
- Collect the solid yellow Mo2(ArNC(H)NAr)4 and allow it to air dry.
- Using CDCl3 measure the 1H NMR spectrum of the product.
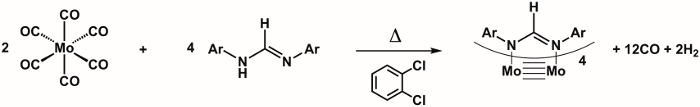
Figure 6. Synthesis of Mo2(ArNC(H)NAr)4, where Ar = p-MeOC6H4.
4. Single Crystal Growth
NOTE: Mo2(ArNC(H)NAr)4 oxidizes slowly in solution. The crystallization solvent should be degassed before use, but rigorous air-free conditions are not necessary to obtain X-ray quality crystals for single crystal X-ray diffraction.
- Degas 10 mL of dichloromethane (CH2Cl2) by bubbling N2 gas through the solution for 10 min (see the "Synthesis of a Ti(III) Metallocene Using Schlenk line Technique" video for a more detailed procedure on purging liquids).
- Make a saturated solution of Mo2(ArNC(H)NAr)4 by dissolving 20 mg of the solid in 2 mL of the degassed CH2Cl2.
- Make a pipette Celite plug by inserting a small piece of Kimwipe into a pipette. Add a small amount of Celite to the pipette.
- Filter the CH2Cl2 solution through the pipette Celite plug into a small 5 mL vial. Help to push the solution through the Celite using a pipette bulb.
- Using tweezers, insert the 5 mL vial into a 10 mL scintillation vial.
- In the outer scintillation vial, add 2 mL of hexanes.
- Tightly cap the scintillation vial and place it on a shelf where it will be undisturbed.
- Allow at least 24 h for single crystal growth (see the "X-ray Crystallography" video in the Essentials of Organic Chemistry series for a more detailed procedure on how to grow single crystals).
- Collect single crystal X-ray data on the sample (see the "Single Crystal and Powder X-ray Diffraction" video for a more detailed procedure on how to collect X-ray data).
Paddlewheel complexes are a class of compounds comprised of two metal ions held in proximity to each other by four bridging ligands. Depending on their properties, paddlewheel complexes are used as catalysts or building blocks for metal-organic frameworks, also known as MOFs.
The M-M bonding in a paddlewheel complex affects the structure and reactivity of the compound, and can be further modified by variation of the metal ion and ligands.
In order to understand these properties, it is crucial to comprehend the electronic structure of the M-M bond in a given paddlewheel complex.
This video will illustrate the principles of M-M bonding, the synthesis and analysis of a dinuclear molybdenum complex, and various applications of paddlewheel complexes.
The M-M bond in a paddlewheel complex can be explained using molecular orbital theory.
When d-orbitals of two transition metals overlap, a M-M bond is formed. Depending on the orbital symmetry, three types of bonds can be created: σ, π, and δ bonds.
If the z axis is assigned to the M-M bond, both dz2 orbitals overlap head-on to form a σ bond. Overlap between two lobes of the dxz or dyz orbitals creates a π bond. Overlap between all four lobes of the dxy or dx2-y2 orbitals creates a δ bond.
The dx2-y2 orbital forms strong M-L bonds and usually does not contribute to M-M bonding. Hence, the maximum bond order achievable in many complexes is four.
Now, let's take a look at the M-M bond in a dimolybdenum complex. First, assign the axes and highest available symmetry.
The z-axis describes the highest rotational symmetry, which is the C4 axis lying along the Mo-Mo bond. Next, assign the x- and y-axis, which lie along the Mo-N bonds.
As seen, the dx2-y2 orbital on each Mo atom is involved in M-L bonding, leaving the dxy, dxz, dyz, and dz2 orbitals for M-M bonding. This can be further described with an MO diagram.
Linear combination of the dz2 orbital on each metal atom results in σ and σ* molecular orbitals, while dxz and dyz orbitals form π and π* MOs. Finally, linear combination of dxy atomic orbitals creates the δ and δ* MOs. Filling the MOs with the d electrons of the Mo centers results in a quadruple bond.
M-M bonds can be measured using X-ray crystallography. To normalize for atomic radius, the formal shortness ratio is calculated with this equation. The FSR describes the ratio of the bond distance in the solid state to the sum of the atomic radii of the individual atoms, and is used to analyze and compare bonds in different metal complexes.
Now that you understand what quadruple bonds are and how to analyze them, let's use this knowledge in a real example.
To begin, combine 6.0 g of p-anisidine and 4.2 mL of triethylorthoformate in a 100 mL round bottom flask with a magnetic stir bar. Attach a distillation head to the reaction flask, and place a beaker at the end of it.
Turn on the stirrer and hot plate. Collect the distilling byproduct ethanol in the beaker, and turn off the heat when ethanol distillation ceases.
Remove the flask from the oil bath and allow the reaction mixture to cool to room temperature. A precipitate should form. If the product does not precipitate, place the flask in an ice bath and scratch the bottom of the flask with a spatula to encourage crystallization.
Recrystallize the product from a minimal amount of boiling toluene. Collect the product by filtration through a fritted funnel and wash with 10 mL of hexanes.
Isolate the white product and allow it to dry in air in recrystallization dish. Lastly, using CDCl3, obtain a 1H NMR of the solid.
Before you start the synthesis, set up the Schlenk Line, ensuring N2 flow and a filled cold trap.
Familiarize yourself with the safety precautions using Mo(CO)6, which is highly toxic, and the Schlenk line techniques.
First, add 1.0 g of the freshly synthesized ligand and 0.34 g Mo(CO)6 to a 100 mL Schlenk flask and prepare the Schlenk flask for the cannula transfer of solvent.
Next, using cannula transfer add 20 mL of degassed o-dichlorobenzene to the Schlenk flask. Fit the Schlenk flask with a condenser connected to N2, and place the flask into a silicone-oil bath. Reflux the reaction for 2 h at 180 °C.
When finished, remove the Schlenk flask from the oil bath and allow the mixture to cool to room temperature. Once cooled, promptly filter the brown solution through a fritted funnel, to reduce the rate of product oxidation in presence of air.
Wash the yellow precipitate with 10 mL of hexanes, followed by 5 mL of reagent grade acetone. Collect the yellow, solid product and allow it to dry on air. Using CDCl3, measure the 1H NMR spectrum of the product.
First, degas the 20 mL of CH2Cl2 to minimize the rate of product oxidation by bubbling N2 through it for 10 minutes. Then, dissolve 20 mg of the product in 2 mL of degassed CH2Cl2 to make a saturated solution.
Next, insert a small piece of a low-lint wipe into a pipette to make a Celite plug. Add a small amount of Celite to the pipette. Filter the saturated solution of product in CH2Cl2 through the plug into a 5 mL vial. Use a pipette bulb to carefully push the solution through the plug.
Using tweezers, insert the 5 mL vial into a 10 mL scintillation vial. Add 2 mL of hexanes to the outer scintillation vial. Cap it tightly and place it on a shelf where the scintillation vial will not be disturbed.
Wait at least 24 hours to allow for single crystal growth, then collect single crystal X-ray data on the sample. Now that all the data is collected, let's take a look at the results.
The ligand exhibits a characteristic peak for the NHC-HN bond at 8.02 ppm. The aromatic peaks integrate to 8H, and the two methoxy groups integrate to 6H total at 3.80 ppm.
In comparison, the singlet for the NHC-HN bond in the product occurs at 8.37 ppm and integrates to 4H. The doublets from the aromatic hydrogens are located at 6.49 and 6.16 ppm with a total integration of 32H. Lastly, the methoxy-groups are found at 3.70 ppm with an integration of 24H.
The two signals in the aromatic region indicate the 4-fold symmetry of the product. Additionally, the solid-state structure is consistent with the D4 point group and features a short Mo-Mo bond of 2.0925(3) Å.
Using the atomic radius of Mo, the FSR value for the M-M bond is calculated to be 0.72, which is consistent with the presence of a M-M quadruple bond.
Paddlewheel complexes, such as the dinuclear molybdenum complex synthesized in this video, display a wide range of properties and thus find application in diverse areas of chemistry.
For example, M-M bonds play an important role in catalysis. The paddlewheel complex Rh2(OAc)4 is a known catalyst for C-H bond functionalization via carbene and nitrene transfer reactions.
In a typical carbene transfer reaction, Rh2(OAc)4 reacts with a diazo compound to generate a Rh2 carbene intermediate. Subsequent insertion of the carbene into a C-H bond generates the product of C-H functionalization and regenerates the Rh2(OAc)4 catalyst.
Metal-organic frameworks, also known as MOFs, are porous compounds made of metal clusters linked together by organic ligands. This type of compound is a subclass of coordination polymers and can form one-, two-, or three-dimensional superstructures.
MOFs are used in many fields. Due to their high porosity and their large surface area per volume, MOFs find applications ranging from catalysts to gas storage and separation.
You've just watched JoVE's introduction to quadruply M-M bonded complexes. You should now understand what quadruple M-M bonds are, how to synthesize paddlewheel complexes, and how to analyze them. Thanks for watching!
Sonuçlar
Ligand ArN(H)C(H)NAr
Yield: 3.25 g (53%). 1H NMR (chloroform-d, 500 MHz, δ, ppm): 8.06 (s, 1H, NHC-HN), 6.99 (d, 4H, aromatic C-H, J = 8.7 Hz), 6.86 (d, 4H, aromatic C-H, J = 9.0 Hz), 3.80 (s, 6H, -OCH3).
Mo complex Mo2(ArNC(H)NAr)4
Yield: 450 mg (57%). 1H NMR (chloroform-d, 500 MHz, δ, ppm): 8.38 (s, 4H, NHC-HN), 6.51 (d, 16H, aromatic C-H, J = 8.8 Hz), 6.16 (d, 16H, aromatic C-H, J = 8.8 Hz), 3.71 (s, 24H, -OCH3).
Table 1. Crystal Data and Unit Cell Parameters
| Empirical formula | C60H70Mo2N8O8 |
| Formula weight (g/mol) | 1223.12 |
| Temperature (K) | 296.15 |
| Crystal system | triclinic |
| Space group | P-1 |
| a (Å) | 10.1446(4) |
| b (Å) | 10.3351(4) |
| c (Å) | 13.9623(6) |
| α (°) | 80.151(2) |
| β (°) | 75.251(2) |
| γ (°) | 82.226(2) |
| Volume (Å3) | 1388.3(1) |
The 1H NMR spectrum of Mo2(ArNC(H)NAr)4 exhibits two signals in the aromatic region, which is consistent with 4-foldsymmetry. The solid-state structure (Figure 7) is consistent with the point group D4 and features a short Mo-Mo bond (2.0925(3) Å). The atomic radii of Mo are 1.45 Å. Therefore, using Equation 1, the FSR value for the M-M bond in Mo2(ArNC(H)NAr)4 is 0.72. This value is lower than that observed for the Mo-Mo quadruply bonded complex Mo(hpp)4 (hpp = 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidinate), which has an FSR value of 0.797, and is consistent with the presence of a M-M quadruple bond3.
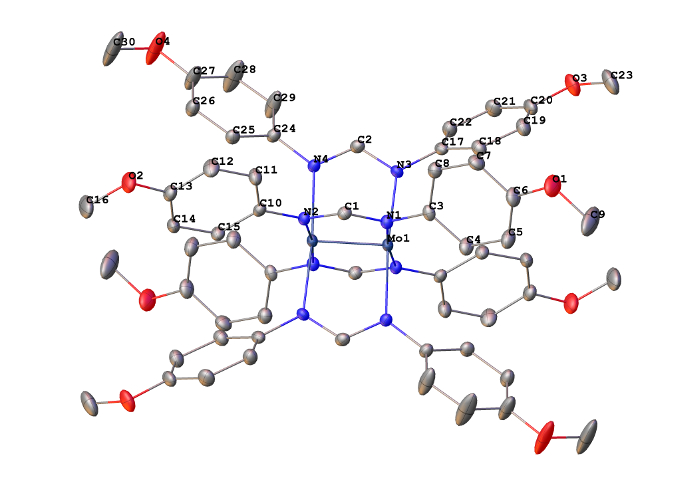
Figure 7. Solid-state structure for Mo2(ArNC(H)NAr)4 with the thermal ellipsoids set at the 50% probability level. Hydrogen atoms are omitted for clarity (Mo navy, N blue, C gray).
Applications and Summary
In this video, we learned about M-M bonding. We synthesized a dinuclear molybdenum complex featuring a quadruple bond. Quadruple bonds consist of three different bond types, including σ, π, and δ bonds. We collected single crystal X-ray diffraction data and observed a short Mo-Mo bond length consistent with a quadruply bonded compound.
Paddlewheel complexes, such as the Mo2 complex prepared here, display a wide range of properties and thus find application in diverse areas of chemistry. For example, M-M bonds play an important role in catalysis: the dirhodium paddlewheel complex Rh2(OAc)4 is a known catalyst for C-H bond functionalization via carbene and nitrene transfer reactions (Figure 8). In a typical carbene transfer reaction, Rh2(OAc)4 reacts with a diazo compound to generate a Rh2 carbene intermediate. Subsequent insertion of the carbene into a C-H bond generates the product of C-H functionalization and regenerates the Rh2(OAc)4 catalyst. The exceptional reactivity of Rh2 catalysts in these reactions has been ascribed to Rh-Rh interaction via the M-M bond. The Rh-Rh bond in the resulting intermediate acts as an electron reservoir; while one metal serves as a binding site for substrate, the second metal center shuttles electron density to and from the active metal center during substrate activation. The d-orbital splitting diagram of the intermediate complex (Rh-Rh core bound to the carbenoid) shows that the frontier d-orbitals are non-bonding with respect to the active Rh center (Figure 9a). The electron density in both the σ and π non-bonding MOs is centered on the nucleophilic carbenoid carbon and the "spectator" Rh center, which is not directly bound to the carbenoid unit (Figure 9b)4.
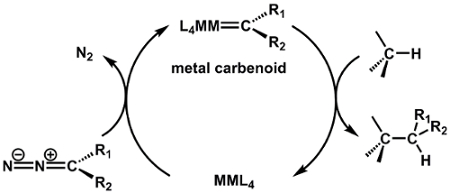
Figure 8. C-H bond functionalization via a metal-carbenoid intermediate.
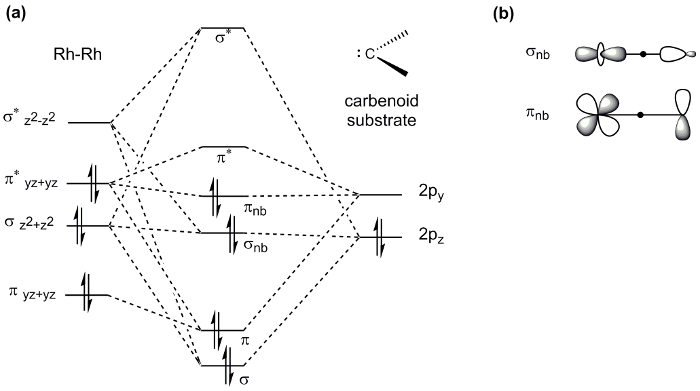
Figure 9. (a) d-orbital MO splitting diagram of the Rh-Rh core in paddlewheel complexes bound to a carbenoid substrate. Note that only orbitals involved in substrate binding are shown. (b) The resulting σ and π non-bonding MOs are filled with electrons. The electron density in those MOs is centered on the carbenoid carbon and the "spectator" Rh center.
Paddlewheel complexes have also been utilized as building blocks in MOFs. MOFs are porous coordination polymers that consist of metal complexes linked together by organic ligands. The resulting one-, two-, or three-dimensional superstructures can be used in a variety of applications ranging from gas absorption (including separation and purification) to catalysis.
Referanslar
- Nguyen, T., Sutton, A. D., Brynda, M., Fettinger, J. C., Long, G. J., Power, P. P. Synthesis of a stable compound with fivefold bonding between two chromium(I) centers. Science. 310(5749), 844-847 (2005).
- Lin, C., Protasiewicz, J. D., Smith, E. T., Ren, T. Linear free energy relationship in dinuclear compounds. 2. Inductive redox tuning via remote substituents in quadruply bonded dimolybdenum compounds. Inorg Chem. 35(22), 6422-6428 (1996).
- Cotton, F. A., Murillo, C. A., Walton, R. A. Eds. Multiple Bonds Between Metal Atoms, 3rd ed. Springer. New York, NY. (2005).
- Nakamura, E., Yoshikai, N., Yamanaka, M. Mechanism of C−H Bond Activation/C−C Bond Formation Reaction between Diazo Compound and Alkane Catalyzed by Dirhodium Tetracarboxylate. J Am Chem Soc. 124 (24), 7181-7192 (2002).
DEŞİFRE METNİ
Paddlewheel complexes are a class of compounds comprised of two metal ions held in proximity to each other by four bridging ligands. Depending on their properties, paddlewheel complexes are used as catalysts or building blocks for metal-organic frameworks, also known as MOFs.
The M-M bonding in a paddlewheel complex affects the structure and reactivity of the compound, and can be further modified by variation of the metal ion and ligands.
In order to understand these properties, it is crucial to comprehend the electronic structure of the M-M bond in a given paddlewheel complex.
This video will illustrate the principles of M-M bonding, the synthesis and analysis of a dinuclear molybdenum complex, and various applications of paddlewheel complexes.
The M-M bond in a paddlewheel complex can be explained using molecular orbital theory.
When d-orbitals of two transition metals overlap, a M-M bond is formed. Depending on the orbital symmetry, three types of bonds can be created: σ, π, and δ bonds.
If the z axis is assigned to the M-M bond, both dz2 orbitals overlap head-on to form a σ bond. Overlap between two lobes of the dxz or dyz orbitals creates a π bond. Overlap between all four lobes of the dxy or dx2-y2 orbitals creates a δ bond.
The dx2-y2 orbital forms strong M-L bonds and usually does not contribute to M-M bonding. Hence, the maximum bond order achievable in many complexes is four.
Now, let’s take a look at the M-M bond in a dimolybdenum complex. First, assign the axes and highest available symmetry.
The z-axis describes the highest rotational symmetry, which is the C4 axis lying along the Mo-Mo bond. Next, assign the x- and y-axis, which lie along the Mo-N bonds.
As seen, the dx2-y2 orbital on each Mo atom is involved in M-L bonding, leaving the dxy, dxz, dyz, and dz2 orbitals for M-M bonding. This can be further described with an MO diagram.
Linear combination of the dz2 orbital on each metal atom results in σ and σ* molecular orbitals, while dxz and dyz orbitals form π and π* MOs. Finally, linear combination of dxy atomic orbitals creates the δ and δ* MOs. Filling the MOs with the d electrons of the Mo centers results in a quadruple bond.
M-M bonds can be measured using X-ray crystallography. To normalize for atomic radius, the formal shortness ratio is calculated with this equation. The FSR describes the ratio of the bond distance in the solid state to the sum of the atomic radii of the individual atoms, and is used to analyze and compare bonds in different metal complexes.
Now that you understand what quadruple bonds are and how to analyze them, let’s use this knowledge in a real example.
To begin, combine 6.0 g of p-anisidine and 4.2 mL of triethylorthoformate in a 100 mL round bottom flask with a magnetic stir bar. Attach a distillation head to the reaction flask, and place a beaker at the end of it.
Turn on the stirrer and hot plate. Collect the distilling byproduct ethanol in the beaker, and turn off the heat when ethanol distillation ceases.
Remove the flask from the oil bath and allow the reaction mixture to cool to room temperature. A precipitate should form. If the product does not precipitate, place the flask in an ice bath and scratch the bottom of the flask with a spatula to encourage crystallization.
Recrystallize the product from a minimal amount of boiling toluene. Collect the product by filtration through a fritted funnel and wash with 10 mL of hexanes.
Isolate the white product and allow it to dry in air in recrystallization dish. Lastly, using CDCl3, obtain a 1H NMR of the solid.
Before you start the synthesis, set up the Schlenk Line, ensuring N2 flow and a filled cold trap.
Familiarize yourself with the safety precautions using Mo(CO)6, which is highly toxic, and the Schlenk line techniques.
First, add 1.0 g of the freshly synthesized ligand and 0.34 g Mo(CO)6 to a 100 mL Schlenk flask and prepare the Schlenk flask for the cannula transfer of solvent.
Next, using cannula transfer add 20 mL of degassed o-dichlorobenzene to the Schlenk flask. Fit the Schlenk flask with a condenser connected to N2, and place the flask into a silicone-oil bath. Reflux the reaction for 2 h at 180 °C.
When finished, remove the Schlenk flask from the oil bath and allow the mixture to cool to room temperature. Once cooled, promptly filter the brown solution through a fritted funnel, to reduce the rate of product oxidation in presence of air.
Wash the yellow precipitate with 10 mL of hexanes, followed by 5 mL of reagent grade acetone. Collect the yellow, solid product and allow it to dry on air. Using CDCl3, measure the 1H NMR spectrum of the product.
First, degas the 20 mL of CH2Cl2 to minimize the rate of product oxidation by bubbling N2 through it for 10 minutes. Then, dissolve 20 mg of the product in 2 mL of degassed CH2Cl2 to make a saturated solution.
Next, insert a small piece of a low-lint wipe into a pipette to make a Celite plug. Add a small amount of Celite to the pipette. Filter the saturated solution of product in CH2Cl2 through the plug into a 5 mL vial. Use a pipette bulb to carefully push the solution through the plug.
Using tweezers, insert the 5 mL vial into a 10 mL scintillation vial. Add 2 mL of hexanes to the outer scintillation vial. Cap it tightly and place it on a shelf where the scintillation vial will not be disturbed.
Wait at least 24 hours to allow for single crystal growth, then collect single crystal X-ray data on the sample. Now that all the data is collected, let’s take a look at the results.
The ligand exhibits a characteristic peak for the NHC-HN bond at 8.02 ppm. The aromatic peaks integrate to 8H, and the two methoxy groups integrate to 6H total at 3.80 ppm.
In comparison, the singlet for the NHC-HN bond in the product occurs at 8.37 ppm and integrates to 4H. The doublets from the aromatic hydrogens are located at 6.49 and 6.16 ppm with a total integration of 32H. Lastly, the methoxy-groups are found at 3.70 ppm with an integration of 24H.
The two signals in the aromatic region indicate the 4-fold symmetry of the product. Additionally, the solid-state structure is consistent with the D4 point group and features a short Mo-Mo bond of 2.0925(3) Å.
Using the atomic radius of Mo, the FSR value for the M-M bond is calculated to be 0.72, which is consistent with the presence of a M-M quadruple bond.
Paddlewheel complexes, such as the dinuclear molybdenum complex synthesized in this video, display a wide range of properties and thus find application in diverse areas of chemistry.
For example, M-M bonds play an important role in catalysis. The paddlewheel complex Rh2(OAc)4 is a known catalyst for C-H bond functionalization via carbene and nitrene transfer reactions.
In a typical carbene transfer reaction, Rh2(OAc)4 reacts with a diazo compound to generate a Rh2 carbene intermediate. Subsequent insertion of the carbene into a C-H bond generates the product of C-H functionalization and regenerates the Rh2(OAc)4 catalyst.
Metal-organic frameworks, also known as MOFs, are porous compounds made of metal clusters linked together by organic ligands. This type of compound is a subclass of coordination polymers and can form one-, two-, or three-dimensional superstructures.
MOFs are used in many fields. Due to their high porosity and their large surface area per volume, MOFs find applications ranging from catalysts to gas storage and separation.
You’ve just watched JoVE’s introduction to quadruply M-M bonded complexes. You should now understand what quadruple M-M bonds are, how to synthesize paddlewheel complexes, and how to analyze them. Thanks for watching!
