Scalable Biomanufacturing Workflow to Produce and Isolate Natural Killer Cell-Derived Extracellular Vesicle-Based Cancer Biotherapeutics
Özet
Natural killer cell-derived extracellular vesicles (NK-EVs) hold promising potential as cancer biotherapeutics. This methodology-based study presents a scalable closed-loop biomanufacturing workflow designed to continuously produce and isolate large quantities of high-purity NK-EVs. In-process control testing is performed throughout the biomanufacturing workflow, ensuring the EVs meet quality standards for product release.
Abstract
Natural killer cell-derived extracellular vesicles (NK-EVs) are being investigated as cancer biotherapeutics. They possess unique properties as cytotoxic nanovesicles targeting cancer cells and as immunomodulatory communicators. A scalable biomanufacturing workflow enables the production of large quantities of high-purity NK-EVs to meet the pre-clinical and clinical demands. The workflow employs a closed-loop hollow-fiber bioreactor, enabling continuous production of NK-EVs from the NK92-MI cell line under serum-free, xeno-free, feeder-free, and antibiotic-free conditions in compliance with Good Manufacturing Practices standards. This protocol-driven study outlines the biomanufacturing workflow for isolating NK-EVs using size-exclusion chromatography, ultrafiltration, and filter-based sterilization. Essential NK-EV product characterization is performed via nanoparticle tracking analysis, and their functionality is assessed through a validated cell viability-based potency assay against cancer cells. This scalable biomanufacturing process holds significant potential to advance the clinical translation of NK-EV-based cancer biotherapeutics by adhering to best practices and ensuring reproducibility.
Introduction
In the 21st century, remarkable advancements have been achieved in the battle against cancer. This is mainly due to the rise of cancer immunotherapeutics, a class of drugs that harnesses the immune system to fight cancer. Natural killer cell-derived extracellular vesicles (NK-EVs) represent promising contenders in the expanding realm of immunotherapy. Integrative to innate and adaptive immunity, NK cells play a crucial role in the body's defense against virus-infected, stressed, and malignant cells. They employ a comprehensive arsenal of anti-cancer machinery to eliminate abnormal cells through cytotoxic means1,2,3. Among these mechanisms is the production and secretion of EVs, nanoscale bilayer structures containing various biomolecules, such as proteins, RNAs, and DNAs, crucial for facilitating intercellular communication4,5,6. NK-EVs emerge as promising cell-free therapeutics due to their unique carrier properties. These include their small size, allowing filter-based sterilization, high biocompatibility, preferential accumulation within tumors, broad cargo delivery spectrum, capacity to overcome biological barriers such as the blood-brain barrier, and minimal toxicity profile. For several reasons, NK-EVs obviate the need for patient lymphodepletion via chemotherapy before administration: 1) conventionally, lymphodepletion is employed to create a more hospitable environment for cell-based therapy, enabling infused cells to proliferate and exert their therapeutic effects; 2) unlike cells, EVs lack the replication capacity and are substantially smaller in scale; 3) EVs operate through distinct mechanisms and exhibit diminished immunogenicity compared to cells5,6,7. Furthermore, NK-EVs consistently exhibited in vitro efficacy against various cancer models and have also shown immunomodulatory effects on immune cells that foster anti-cancer responses8,9. In vivo results corroborate these findings, showcasing cancer regression following NK-EV treatment and negligible toxicities10,11,12. Therefore, NK-EV-based therapeutics hold great promise to address the challenges of treating cold, immunologically inert solid tumors13,14,15,16,17.
Our recent study addresses a significant bottleneck to the clinical translation of NK-EVs through biomanufacturing7. The article presents a proof-of-concept for a cost-effective and scalable biomanufacturing workflow of NK-EVs meticulously designed to ensure in-process quality control testing. This approach continuously produced large quantities of high-purity NK-EV-based cancer biotherapeutics, with thorough product characterization conducted according to the MISEV2018 guidelines18. The scalability of the biomanufacturing workflow can be achieved by increasing cartridge size or having multiple bioreactors running in parallel. Similarly, the scalability of the EV isolation workflow can be easily attainable using techniques like Fast Protein Liquid Chromatography (FPLC) based size-exclusion chromatography (SEC), ultrafiltration (UF), and filter-based sterilization. The closed-loop hollow-fiber bioreactor (HFB) system grew the IL-2-self-sufficient NK cell line (NK92-MI cells) without requiring serum supplementation, a feeder system, and antibiotics. This was accomplished using a commercially available chemically defined and xeno-free medium (a GMP version is now commercially available). As a result, large quantities of NK cells (109 viable cells) and NK-EVs (1012 EVs) were successfully produced within 5 – 7 days using a single medium-size bioreactor cartridge, with both products extensively characterized. Throughout the biomanufacturing process, cell health was monitored daily using quantifiable metrics such as pH, glucose, and lactate levels, along with visual indicators such as media color and any sign of contamination, which are essential predictors of cell and EV quality. Post-harvest evaluation of NK cell viability and functionality generated in the HFB system, particularly cytotoxicity, revealed a significant enhancement compared to flask-based cultures7. Likewise, purified NK-EVs exhibited a high purity profile, devoid of bacteria, mycoplasma, common viral entities, and cellular components, and with negligible endotoxin levels. Importantly, purified NK-EVs constituted over 99.9% of all nanoparticles found in the final product7. Lastly, these purified NK-EVs retained key NK characteristics, including surface markers (CD2, CD45, CD56), cytokine payload (GzmB, PFN, IFN-g), and demonstrated potent cytotoxicity against leukemic K562 cells, the gold-standard line for assessing NK cell cytotoxicity7.
The present protocol details the scalable biomanufacturing workflow discussed above. It elucidates the methodology for isolating NK-EVs produced using FPLC-SEC coupled with UF and filter-based sterilization. Additionally, the protocol describes pivotal steps, including product characterization using nanoparticle tracking analysis (NTA), quality assessment using various tools (protein/dsDNA quantification and microbial testing), and functional validation of the purified NK-EV product against cancer cells by cell viability assay. Typically, this workflow yields 1.0 – 1.5 mL of NK-EV product with an average concentration of 1.18 x 1012 EVs/mL7, totaling a minimum of 1 x 1012 EVs based on approximately 40 mL of EV-rich CM. This process allows product release for various downstream applications, such as investigatory, preclinical, and multi-omics (proteomics, transcriptomics, genomics, metabolomics, lipidomics, and epigenomics) studies demanding high quantities of high-quality EVs while holding potential for clinical translation, with demonstrated reproducibility.
Protocol
1. NK-EV biomanufacturing from NK92-MI cells using a closed-loop bioreactor
NOTE: NK-EVs are manufactured using a scalable biomanufacturing workflow that adheres to Good Manufacturing Practices (GMP) and utilizes the NK92-MI cells (see Figure 1). Our recent publication has detailed insights into the biomanufacturing procedure and NK-EV products' identity and safety profiles7.

Figure 1: Biomanufacturing of natural killer cell-derived extracellular vesicles (NK-EVs) in a closed-loop hollow-fiber bioreactor (HFB) with scalable isolation workflow. Schematic representation of the biomanufacturing workflow to generate large quantities of high-purity NK-EV products. IL-2 self-sufficient NK92-MI cells are seeded into a closed-loop HFB cartridge and cultured under serum-free (SF), xeno-free (XF), feeder-free, and antibiotic-free conditions, where they are grown for continual EV-rich conditioned medium collection. NK-EV isolation from EV-rich CM is performed by Fast Protein Liquid Chromatography-based size exclusion chromatography (FPLC-SEC) coupled with ultrafiltration (UF). NK-EVs are characterized and assessed through multiple assays, and their functionality against K562 leukemia cells is evaluated using a viability potency assay. This figure has been modified from7(created with Biorender.com). Please click here to view a larger version of this figure.
- Starting from 1 – 5 x 106 NK92-MI cells and maintaining a cell density between 3 – 8 x 105 cells/mL, culture the cells in T25 – T175 flasks using a pre-warmed culture medium. Incubate at 37 °C with 5% CO2 (see Table of Materials). Replace the medium every 2 – 3 days until 1 x 108 NK92-MI cells are produced of at least 70% viability.
NOTE: Keep 1/5th-1/3rd of the conditioned medium (CM) when reseeding the cells, as it contains favorable growth factors. - Perform medium HFB cartridge preparation and NK cell inoculation as described below.
NOTE: All manipulations should be performed inside a class II biosafety cabinet to ensure and maintain sterility. Before moving the cartridge system into the biosafety cabinet, generously spray with 70% ethanol, paying special attention to the reservoir bottleneck and the syringe connections.- Prepare the HFB cartridge according to the manufacturer's instructions19 (see Table of Materials; see Figure 2) as described below.
- Wrap Luer Lock connections with wax film and adjust the pump flow rate according to the manufacturer's instructions19. Condition the HFB cartridge by allowing 150 mL of sterile phosphate-buffered saline (PBS; see Table of Materials) to circulate for at least five days.
- To remove the air from the extracellular capillary space (ECS; volume is approximately 29 mL), inject approximately 40 mL of PBS through the left ECS port and allow the air to escape through the right ECS port. While doing that, close the left and right end port clamps. Ensure the syringe is always connected to the left and right ECS ports.
- Once completed, put the cartridge on the flow pump (see Table of Materials) inside the incubator set to 37 °C and 5% CO2. Ensure there are no leaks after a few days of circulation.
- Replace the PBS with 150 mL of culture medium in the reservoir bottle and the ECS 2 days before seeding cells into the cartridge. Repeat the previous conditioning steps (step 1.2.1.) using the culture medium but for 2 days of circulation.
- Before seeding cells, replace the contents in the reservoir bottle and the ECS with 250 mL of fresh culture medium.
- Acquire the culture flask from the incubator and transfer the cells to a 50 mL tube. Spin at 300 x g for 5 min. Resuspend the cell pellet using 21 mL of culture medium.
- Prepare two aliquots of 20.5 µL each from the cell suspension for cell counting on an automated cell counter (see Table of Materials). To each 20.5 µL cell suspension aliquot, add an equal amount of AO/PI dye (see Table of Materials) and mix up and down at least 10x.
NOTE: We do not recommend Trypan Blue for accurate NK cell counting. Alternatively, use a hemocytometer for manual counting. - Load 20 µL into each counting chamber of the counting slide and perform automated cell counting using the appropriate program. Calculate the average live cell concentration and note viability.
- Mix the NK cell solution a few times before aspirating it using a 20 mL syringe and an 18 G needle to maintain sterility. This solution should contain approximately 1 x 108 live NK cells in approximately 20 mL or about 5 x 106 cells/mL.
- After removing the needle from the syringe, gently inject the NK cells into the cartridge through the left ECS port. To ensure uniform cell dispersion throughout the cartridge, gently reciprocate the cell solution at least 10x using the syringes connected to the left and right ECS ports.
NOTE: the solution should have equal turbidity across both syringes, with the left and right end ports closed. - Open the left and right end port and inject what remains within the syringes. Close the left and right ECS ports using the clamps.
- Transfer the cartridge to the incubator and let it sit for 30 min before properly installing it on the flow pump. Leave the cartridge in for biomanufacturing. Adjust the flow rate according to the manufacturer.
- To monitor cell health metrics, acquire a 0.5 mL aliquot of medium daily from the thoroughly mixed medium in the reservoir bottle and store it at -20 °C after verifying glucose and pH levels. L-lactate levels can be verified later (see Table of Materials).
- Replace the medium in the reservoir (250 – 500 mL) every 1 – 2 days to maintain the glucose content above 50% of the initial levels found in the medium and pH above 7.0 (range from 7.0 – 8.0).
- Prepare the HFB cartridge according to the manufacturer's instructions19 (see Table of Materials; see Figure 2) as described below.
- Perform NK-EV-rich CM collection daily after 1 day of rest when first seeding the cartridge as described below.
- Move the cartridge system into the biosafety cabinet. Gently inject approximately 21 mL of culture medium through the left ECS port to push an equivalent volume of EV-rich CM through the right ECS – do not mix (see Figure 3).
NOTE: Always use new plasticware to prevent contamination. - Transfer EV-rich CM solution into a 50 mL tube and centrifuge at 300 x g for 5 min. Meanwhile, move the cartridge system back into the incubator on the flow pump.
- Transfer the supernatant to a new tube and centrifuge at 2000 x g for 10 min. Again, transfer the supernatant into a new tube. Then, aliquot the EV-rich CM equally across 3, 50 mL tubes (~7 mL/tube) and store at −80 °C until further processing.
NOTE: Sequentially harvested EV-rich CM is pooled across these three tubes, generating three technical replicate sample tubes.
- Move the cartridge system into the biosafety cabinet. Gently inject approximately 21 mL of culture medium through the left ECS port to push an equivalent volume of EV-rich CM through the right ECS – do not mix (see Figure 3).
- Perform HFB-NK cell harvest to continue producing EV-rich CM using the same HFB cartridge as described below.
NOTE: NK cells can be harvested from the HFB's ECS by performing the HFB-NK cell harvest protocol" once the cartridge reaches confluence (maximum of 1 x 109 cells). This happens after 5 – 7 days for each lot or when the glucose content is found to be below the limit of detection of the glucose meter (e.g., no reading or readings of ~ 0) for 2 consecutive days. If this is the final cell harvest, the medium can be substituted for PBS to flush the cartridge and retrieve the cells.- Harvest EV-rich CM exactly as detailed above in step 1.3.
- Inject approximately 50 mL of the medium through the left ECS port. To ensure homogenous cell dispersion throughout the cartridge, gently push back and forth the cell solution using the syringes connected to the left and right ECS ports at least 10x to loosen up cells before ultimately pushing and collecting them with a syringe through the right ECS port. Transfer the harvested EV-rich CM into a 50 mL tube. Set aside at 37 °C (water bath or incubator) for now.
NOTE: The push-back action helps to dislodge the cells before they are fully expelled and collected by a syringe through the right ECS port. The solution should have equal turbidity across both syringes, with the left and right end ports closed. Tapping on the bioreactor cartridge (physical disturbance) can help preemptively dislodge the cell cluster at the bottom of the cartridge. Aggressive back-forth mixing of the cell suspension can negatively affect the viability of the recovered cells. Care and patience should be applied to maximize viability. - Repeat the last step 2x. In total, 150 mL of cell suspension should be recovered. Centrifuge at 300 x g for 5 min. Discard supernatant.
- Resuspend both cell pellets in 20 mL of fresh medium each and combine them. Collect two aliquots of 20.5 µL each of the cell suspension for cell counting on an automated cell counter (see Table of Materials).
NOTE: Typically, numerous cell dilutions using PBS as diluent are required to fall within the cell counter's dynamic range. - To the 20.5 µL cell suspension aliquot, add an equal amount of AO/PI dye (see Table of Materials) and mix up and down at least 10x. Load 20 µL into each counting chamber of the counting slide and perform automated cell counting using the appropriate program.
- Average the live cell concentration of all dilution-corrected counts, determine the total amount of live cells, and record the viability. As detailed above, to continuously produce EV-rich CM using the same bioreactor cartridge, reseed 1 x 108 HFB-produced NK cells.
NOTE: If desired, HFB-produced NK cells can be stored using a cryopreservation freezing medium and a freezing container to control the freezing rate (see Table of Materials).

Figure 2: Hollow-Fiber Bioreactor (HFB) system component and set-up. The reservoir bottle (1) contains the complete medium that circulates through the bioreactor cartridge (2) by the action of a peristaltic pump (not shown) acting on the pump tubing (3). Cells are introduced into the extracellular capillary space (ECS) through the left (4) and right (5) ECS side ports. Once the ECS slide clamps are closed, the left (6) and right (7) end side ports are open to allow the medium to circulate throughout the system. Notice the addition of wax film on the Luer Lock connection near the reservoir cap of the medium bottle to prevent potential contamination. Please click here to view a larger version of this figure.

Figure 3: Schematic representation of the NK-EV isolation process. After daily collection of EV-rich conditioned medium (CM), the solution was differentially centrifugated to remove cells (first spin at 300 x g for 5 min) and cellular debris (second spin at 2000 x g for 10 min). Cleared EV-rich CM was stored at -80 °C until further processing. Once ready for NK-EV isolation, frozen EV-rich CM is thawed and centrifuged one more time to ensure the removal of cellular debris (third spin at 10,000 x g for 30 min). Then, the EV-rich CM is treated for 2 – 4 h at 37 °C with endonuclease to digest nucleic acids considered host cell contaminants. Next, the EV-rich CM is processed by Fast Protein Liquid Chromatography-based size-exclusion chromatography (FPLC-SEC) for EV purification using a bimodal resin. Eluted fractions of approximately 10 – 15 mL are combined and filtered with 0.22 µM filters to ensure the sterility of the final NK-EV product. Ultrafiltration allows the product to be concentrated by a factor of about 35 – 50X, yielding a guaranteed concentration of over 1 x 1012 EVs/mL, totaling 1.0 – 1.5 mL. This figure has been modified from7(created with Biorender.com). Please click here to view a larger version of this figure.
2. NK-EV purification by FPLC-SEC coupled with UF and filter-based sterilization
- Prepare the following solutions and filter them twice using a 0.1 µm filter (see Table of Materials): water (conductivity of 0 mS/cm), PBS: 50 mL of 10x PBS + 450 mL of water (conductivity around 14.7 mS/cm), 20% ethanol, cleaning solution (0.5 M NaOH and 30% isopropyl alcohol in water).
- Perform FPLC system initiation according to manufacturer's instructions (see Table of Materials). Perform pre- and post-run clean-in-place (CIP) steps according to manufacturer's instructions. Wash all lines and the column resin and rinse using double-filtered (DF) water, cleaning solution, DF-water, and DF-PBS.
NOTE: It is worth noting that CIP can be done on another day if needed. - Use a chromatography column packed with a bimodal resin (see Table of Materials) with a bed height of 20 cm. Make connections using the drip-to-drip method to ensure no air is introduced inside the column.
- Set up the fraction collector with appropriate collection tubes and change the fractionation settings to the desired collection volume (e.g., 15 mL). Place enough tubes and two additional tubes to collect the entire sample volume.
- Carry out sample preparation as described below.
- Take 40 – 80 mL of EV-rich CM from the -80 °C freezer and thaw quickly at 37 °C. Load the sample into the ultracentrifuge and spin at 10,000 x g for 30 min at 4 °C.
NOTE: Tubes must be balanced accurately by weight, not by volume. - After spinning, collect the supernatant and transfer it to a new tube. To reduce dsDNA levels, treat the EV-rich CM with 50 U/mL of endonuclease and 1.5 mM of MgCl2 (see Table of Materials). Incubate for 2 – 4 h in an incubator (37 °C), allowing moderate mixing.
- Take 40 – 80 mL of EV-rich CM from the -80 °C freezer and thaw quickly at 37 °C. Load the sample into the ultracentrifuge and spin at 10,000 x g for 30 min at 4 °C.
- Once the system (lines and column) is ready for EV isolation, load the EV-rich CM into a 60 mL syringe and connect it to the sample line. Start the system by clicking Manual Run and set the flow rate to 0. Follow the software prompts to save the run pre-emptively, then click Start.
- Select Line B (DF-PBS) and run at a flow velocity of 150 cm/h (flow rate of 2.0 mL/min). Make sure the solution runs through the column.
- Once the conductivity stabilizes, press Auto Zero UV. Change the flow path to direct the sample to the waste bottle before the column. Make sure no bubbles are introduced into the system.
- After a maximum of 5 – 20 s, direct the sample to the column. Click Fractionation once the UV readings reach approximately 230 mAU.
- Once the sample is completely injected through the system, switch the buffer system over to DF-PBS (at the sample valve) to continue purification. Click Fractionation again when the UV value reaches approximately 1600 mAU.
NOTE: This corresponds to the intersection between UV readings and conductivity readings. Longer fractionation only dilutes the retentate without increasing EV yield. - Combine all the fractions (diluted NK-EVs) and store them at 4 °C until ready for filter-based sterilization and ultrafiltration (UF).
- Continue running DF-PBS until the UV value reaches approximately 1000 mAU. After this, stop running and save the chromatogram as a PDF document.
3. NK-EV product filter-based sterilization and concentration by UF
- Cool down the centrifuge to 10 °C. Sanitize every component of the UF apparatus (see Table of Materials) by rinsing with 20 – 30 mL of 90% ethanol. Spin at 4000 x g for 5 – 10 min.
NOTE: The filter is made of 10 kDa MWCO regenerated cellulose. - Discard the flow through and then repeat the rinse using sterile PBS to equilibrate the device. Repeat 2x in total.
- To maximize sterility, filter the diluted NK-EV solution using a 0.22 µm syringe filter pre-wet with DF-PBS (see Table of Materials). Collect the filtrate directly into the sterilized concentration apparatus.
- Spin at 4000 x g for 15 – 40 min (spin time is sample dependent). Mix the solution within the top filter compartment using a serological pipette after spinning. Spin at 4000 x g for an additional 10 min.
NOTE: The mixing step is optional as it simply eases the concentration step by preventing the membrane from being clogged by EVs. - Temporarily set aside the flow-through and collect the NK-EV sample by inverting the filtration device and attaching it to the collecting device.
- Spin at 2000 x g for 2 min. Transfer the purified NK-EV product into a 2 mL tube. Store the purified NK-EV product at 4 °C for short-term use (≤ 7 days) or frozen at -20 °C for long-term use.
4. NK-EV characterization by Nanoparticle Tracking Analysis (NTA)
- Prepare the solution and filter it twice at 0.1 µm (see Table of Materials): water, PBS, cleaning solution (10% bleach (CAUTION) in water).
- Initiate the NTA system according to the manufacturer's instructions. Similarly, perform the pre- and post-run clean-in-place (CIP) steps. Wash all lines and rinse using double-filtered (DF) water, cleaning solution, and DF water. Equilibrate the lines using DF-PBS.
- Verify the flow cell and check for air bubbles. Remove bubbles if present. Once clear, carefully re-insert the flow cell into the NTA instrument.
NOTE: Although not recommended by the manufacturer, very difficult-to-remove air bubbles can easily be removed by rinsing with 20% ethanol and then DF-water. - Once the flow cell is in place and the door is closed, click Start Camera. With the lines filled with DF-PBS, the screen should show an absolute minimum number of particles.
- Change capture settings to a screen gain of 2 and a camera level of 14. Also, turn on the heater to temperature-stabilize the flow cell.
- Click Standard measurement to create a script under the SOP tab for collecting one capture over 1 min at a flow rate of 30 particles/frame and 23 °C.
- Just below, add the folder and file name to the pathway name to save the data.
- Prepare dilutions of the purified NK-EV product using DF-PBS in advance. When running NTA, accurate quantification requires 30 – 80 particles/frame.
- Vortex the sample before loading it into the syringe (see Table of Materials).
- Carefully connect the 1 mL acquisition syringe to the instrument loading line. No air should be present as it will negatively affect the acquisition and analysis. Slowly push half of the sample through, leaving around 0.5 mL in the syringe.
- Once particles are visible on the screen, focus the camera to have a maximum of one halo around each particle. Click Infuse under the Hardware tab at a rate of 1000 for 5 s. Then, bring it down to a rate of 30.
- Press Run Script and follow the prompts. The software will ensure the temperature is set and ask if the settings are correct. Click Yes and follow the software prompts.
- After completing the capture, click İptal when the software asks to process or export files. Click Infuse under the Hardware tab at a rate of 1000 for 10 – 15 s. In the meantime, turn back on the heater and the camera. Then, bring the rate down to 30 until the particles move.
- Gather four more captures by repeating the previous steps. Once a total of five captures have been recorded per dilutions, perform analysis after importing all five captures.
- Select the files to be processed by highlighting them. Click Process Selected Files. Under the Process tab, adjust the analysis settings to a screen gain of 2 and a detection threshold of 15.
Settings are sample-dependent; ensure that 30 – 80 particles per frame are visible. - Check and click OK for analysis.
- Once the files are processed, the software will ask to export them. Click Yes without clicking additional boxes or click Export.
- Repeat for all EV dilutions or samples. Shut down the NTA instrument after all samples are completed and CIP is done.
5. Quality assurance testing
- Perform microbial testing using two tests: 1) a small aliquot of purified NK-EVs is spiked into autoclaved LB broth medium, and 2) a small aliquot of purified NK-EVs is used for mycoplasma PCR detection (see Table of Materials).
- Test 1: culture LB medium at 37 °C for up to 5 days of with positive and negative controls included. Record the OD600, if needed.
- Test 2: perform mycoplasma PCR detection according to the manufacturer's protocol.
- Quantify protein and dsDNA on purified NK-EV dilutions using fluorometer-based assays as per manufacturer's instructions (see Table of Materials).
6. Potency evaluation of NK-EV treated cancer cells using a validated highly sensitive resazurin-based cell viability assay 20
- Culture human K562 leukemia cells using RPMI-1640 with 10% heat-inactivated FBS for a few days before performing the potency assay (see Table of Materials). Maintain density between 2 – 8 x 105 cells/mL and replace media every 2 – 3 days.
- Acquire a 96-well flat-bottom plate (see Table of Materials) and pre-emptively add the volume of assay medium (supplemented with 5% EV-depleted FBS) required for normalization purposes (see Table of Materials). The final volume is 150 µL/well.
NOTE: Use a repeater pipettor to reduce well-to-well variation. - Acquire the cell culture and transfer the cells to a tube. Spin at 300 x g for 5 min. Resuspend the cell pellet into a single-cell solution using 2 – 5 mL of assay medium.
- Collect an aliquot of 20.5 µL of the cell suspension for cell counting on an automated cell counter (see Table of Materials).
- To the 20.5 µL cell suspension aliquot, add an equal amount of AO/PI dye (see Table of Materials) and mix up and down at least 10x.
NOTE: We recommend AO/PI for accurate cell counting. Alternatively, use a hemocytometer for manual counting. - Load 20 µL into each counting chamber of the counting slide and perform automated cell counting using the appropriate program. Average the live cell concentration and record the viability.
- Transfer approximately 1 x 106 cells into a secondary tube. Dilute the cells to precisely 7 mL of assay medium and repeat cell counting. The concentration should be approximately 1.2 – 1.5 x 105live cells/mL.
- As detailed above, adjust the single-cell suspension concentration to 1 x 105 live cells/mL and repeat cell counting if needed.
NOTE: The coefficient of variation between technical duplicate counts should be less than 25%; typically, it is less than 5% with AO/PI counting. - Once the desired concentration is achieved, transfer 50 µL (± 1 µL) of this solution into each well to get as close as possible to 5000 cells/well (4900 – 5100 cells/well). Prepare technical triplicates for each assay condition and use a repeater pipettor to reduce well-to-well variation.
- Transfer the plate to an orbital shaker (350 – 500 RPM) for 2 min. Transfer cells back to the incubator until ready to proceed with NK-EV treatment.
- Prepare the required NK-EV dilutions (1:5, 1:10, and 1:100) using an assay medium.
- From these dilutions, test the following EV concentrations: 1 x 108, 5 x 108, 1 x 109, 5 x 109, 1 x 1010, 5 x 1010 and 1 x 1011 particles/mL. The dosing volume is limited to 20% of the total assay volume.
- Once ready, transfer the required volume of a given dilution to the wells requiring a desired EV concentration for treatment. Add 15 µL of 10x Triton-X to the positive control well (see Table of Materials). The final well volume should be normalized to 150 µL.
- Add the plate to an orbital shaker (350 – 500 RPM) for 2 min. Incubate the cells at 37 °C in the 5% CO2 incubator for 3 h.
- Pre-warm the plate reader (see Table of Materials) at 37 °C and load the following script: 37 °C (reduces temperature-related variation), 450 RPM mixing for 1 min (ensures sample homogeneity), and read.
- Add 15 µL of the resazurin-based reagent to each well (see Table of Materials). Protect the reagent from light and use a repeater pipettor to reduce well-to-well variation.
- Add the plate to an orbital shaker (350 – 500 RPM) for 2 min. Transfer the plate to the incubator and incubate for 60 min. Remove air bubbles using an ethanol-dipped pipette tip. Read plate using an excitation of 560 nm and an emission of 590 nm.
- Data analysis: Average technical replicates were averaged and correct for background before performing a dose-response analysis using a non-linear regression for the inhibition effect showing the log(inhibitor) vs. normalized response-variable slope without constraint. Record the Hillslope and EC50 values.
Representative Results
NK-EVs possess inherent cytotoxic functions and have demonstrated high efficacy against various cancer models. However, there needs to be more standardization among current studies regarding a biomanufacturing workflow suitable for the large-scale production of NK-EVs6,21. Our previous study described the feasibility of a closed-looped hollow-fiber bioreactor (HFB) system to produce large quantities of high-purity NK-EV products7. As a follow-up, this protocol-based study details the biomanufacturing workflow and demonstrates its reproducibility by producing and isolating the NK-EV product (Figure 1). Furthermore, essential product characterization and validation are required before product release is performed, whereby new and original data are presented in this study.
The HFB system was selected for NK-EV production due to its ease of use, reliability, scalability, and GMP compliance7. In reference to the HFB system set-up, the NK cells are injected through the left ECS port and seeded into the bioreactor cartridge (Figure 2). At the same time, the media bottle is connected to the HFB through the side ports, and the media is allowed to flow throughout the system. The NK cells are cultured in serum-free, xeno-free, feeder-free, and antibiotic-free medium, where the media is replaced when the glucose content falls below 50% to maintain and maximize cell health over time. CM is collected daily, processed through differential centrifugations, and kept frozen (-80 °C) until ready for further processing. Afterward, EV isolation is conducted through a combination of differential centrifugations and FPLC-SEC coupled with UF and filtration (Figure 3). This results in a concentrated and sterile NK-EV product with a final volume of approximately 1.0 – 1.5 mL. A representative chromatogram of the FPLC-SEC isolation of NK-EVs is provided (Figure 4). Before FPLC-SEC processing, the NK-EV-rich CM is treated with endonuclease, significantly reducing dsDNA levels, a potential host (NK) cell contaminant7. Thus, the described EV isolation workflow removes cellular debris and RNA/DNA contaminants from the NK-EV product, which is essential for ensuring a low and unwanted immunogenic potential and that the final product is suitable for downstream studies.

Figure 4: NK-EV isolation chromatogram generated during Fast Protein Liquid Chromatography size Exclusion. The blue line represents the absorbance (mAU; maximum reading of 2000 mAU), the red line represents the conductivity, the red text represents the run log, and the gray shaded area represents the fractionated NK-EVs (denoted by fractions T2 – T7). Please click here to view a larger version of this figure.
Following isolation, basic NK-EV characterization and quality assurance testing are used to evaluate if the NK-EV product can be released for further downstream experimentation. NK-EV product particle size range and concentration are measured using nanoparticle tracking analysis (NTA), with sizes ranging from 76.30 – 174.30 nm in diameter (D10 of 78.38 ± 2.07 nm, D50 of 106.72 ± 2.43 nm, and D90 of 169.80 ± 4.17 nm) and an average concentration of 1.39 x 1012 EVs/mL (Figure 5A-B). Additionally, fluorometer quantification showed a protein and dsDNA concentration of 298.90 ± 66.62 mg/mL and 225.60 ± 37.7 ng/mL for the final product, respectively (Figure 5C-D). This corresponds to an average ratio of 5.06 x 106 EV/µg of protein and 6.16 x 1012 EV/µg of DNA. Microbial and mycoplasma testing both returned negative results (data not shown). These results are consistent with the characterization of NK-EVs from previous work7. The earlier publication7 also provides a further in-depth characterization of the NK-EV products following the MISEV guidelines (i.e., TEM, western blot, endotoxin level, viral entities, and flow cytometry for surface antigens and cytokines).
Lastly, the NK-EV product's functionality (i.e., cytotoxicity against cancer cells) was assessed using a validated highly sensitive resazurin-based cell viability assay following NK-EV treatment against leukemic cell line K5627,20. K562 cell treatment with NK-EVs for 3 h produced a dose-dependent effect on cell viability, corresponding to an EC50 of 9.33 x 109 EVs/mL (i.e., the dosage that corresponds to the killing of 50% of the cell population; Figure 6A-B). Thus, following the outlined product release criteria, the NK-EV product is deemed suitable for further experimentation.

Figure 5: Purified NK-EV product characterization. (A) NK-EV product size distribution measured by NTA, shown as mean from 5 independent experiments, each with 10 technical replicates (5 video captures x 2 dilutions). (B) NK-EV particle product concentration (particles/mL) measured by NTA, presented as mean ± SD from 5 independent experiments, each with technical duplicates. (C) NK-EV product protein concentration (mg/mL) was measured by using a fluorometer, presented as mean ± SD from 5 independent experiments, each with technical triplicates. (D) NK-EV product dsDNA concentration (ng/mL) measured by using a fluorometer, presented as mean ± SD from 5 independent experiments, each with technical triplicates. Please click here to view a larger version of this figure.
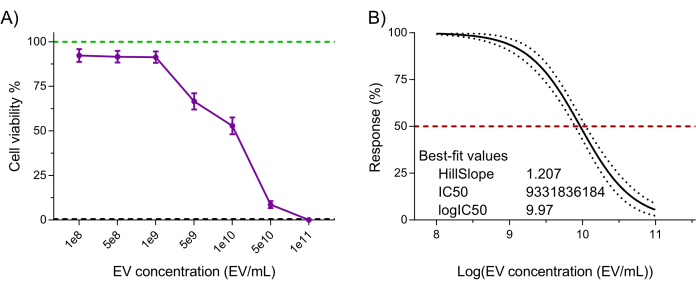
Figure 6: Purified NK-EV product functional validation. NK-EVs demonstrate a dose-dependent cytotoxicity against human K562 leukemia cells treated at various NK-EV concentrations for 3 hours using a highly sensitivity resazurin-based cell viability assay. (A) Normalized assay readouts (green line represents the untreated K562 leukemia cell control, and the black dashed line represents lysed K562 leukemia dead cell control; detergent-treated). Data are shown as mean ± SEM from 11 independent experiments with technical triplicates. (B) EC50 curve analysis with a variable slope for NK-EV treatment with 95% confidence interval/prediction bands (red dashed line represents 50% response). Please click here to view a larger version of this figure.
Discussion
Several studies suggest that NK-EVs possess vast potential as an anti-cancer therapeutic4,5,7,9,16,22,23,24,25,26,27,28,29,30. However, a scalable GMP-compliant biomanufacturing system capable of yielding large quantities of high-purity NK-EVs is required for further pre-clinical testing and future clinical applications. To address this issue, a previous study used a closed-looped HFB system to continuously produce NK cells and NK-EV-rich CM suitable for downstream experimentation. Due to their 3D design, HFB systems closely reflect the conditions of the vascular system and possess an incredibly high surface-area-to-volume ratio, permitting upwards of a billion cells to remain in culture, ultimately leading to improved EV production7,31,32. Importantly, this work was the first to ever report using an HFB system for culturing NK cells, likely due to the cell line IL-2 self-sufficiency7.
Additional steps must be taken to ensure the sterility of the HFB system and the production of high-purity NK-EVs. These precautions are especially crucial in the absence of a sterile, clean room, which may be the case for several research facilities. Before entering the biosafety cabinet, the HFB system is meticulously sprayed with 70% ethanol to disinfect all external surfaces. Additionally, wax film is wrapped around all Luer Lock connections to minimize the risk of contamination. This is particularly important as this biomanufacturing workflow does not use antibiotics, which are known to affect the biochemical profile of cell and cell-derived products33. Various metrics were used to assess cell health during cell product biomanufacturing. For example, daily assessments of the reservoir media's pH, glucose, and lactate levels were conducted as these are vital cell health surrogates for monitoring. In addition to quantitative assessments, qualitative observations of the HFB system (e.g., media color and visual signs of contamination such as turbidity) are also helpful for monitoring cell health. Cell counts on daily retrieved CM have not been found to be a representative metric of viability for the health of the culture (data not shown). This is likely a result of dead cells retrieved during CM sampling that were found within the tubing where media was not allowed to circulate (the small section between the ECS and the ECS syringe port), thereby undervaluing the viability of the overall cell culture. Only harvested NK cells produced by the HFB at the end of a production lot can provide a reliable metric of the culture's health. These cells consistently showed viability values above 70% across production lots7. Together, these quality assessment methods ensure the continuous production of high-purity NK-EVs.
Several isolation techniques have been developed to purify and isolate EVs34. One method, SEC, utilizes a column packed with a porous material – resin – allowing for molecule separation based on size discrimination. Here, the larger EVs are eluted through the column faster; this method is known as flow-through purification based on size exclusion. At the same time, smaller contaminants (dsDNA, free-floating proteins like endonuclease, salts, phenol red, etc.) are left behind and further retained within the resin by electrostatic forces (i.e., a bimodal resin was used). SEC-based processing removes non-EV-bound proteins while maintaining the original EV structure and functionality35,36. Furthermore, SEC-based purification is easily scalable without compromising the high yield and purity, making it a suitable choice for isolating NK-EVs for biotherapeutic uses. Despite these advantages, SEC has some drawbacks, such as the relatively diluted flow-through (eluent); hence, UF is required for product concentration, but it also permits buffer exchange. The non-sterile UF apparatus is rinsed with 70% ethanol and PBS and kept in the biosafety cabinet prior to use to ensure sterility. Typically, the flow-through can be concentrated to 35x-50x of the initial volume while removing small molecules that could have made their way into the eluent. Differential centrifugation and endonuclease treatment are performed before FPLC-SEC coupled with UF to remove residual cells, cellular debris, and long strands of antigenic dsDNA7.
Following NK-EV product isolation, characterization, and functional validation are performed per the guidelines in MISEV2018 and MISEV2023 to determine the product's suitability for further use6,18. Each isolation yields 1.0 – 1.5 mL of high-purity NK-EV product at a minimum concentration of 1 x 1012 EVs/mL, with an average concentration of 1.39 x 1012 particles/mL. Previously, Gupta et al. determined that the median EV dosage in vivo is 3.37 x 108 EVs/kg of body weight of mice37. Treating with the median dosage would require 8.43 x 106 EVs/mouse with a body weight of 25 g, a value far below the guaranteed minimum (1 x 1012 particles/mL) obtained through this workflow. Thus, the described biomanufacturing workflow can produce more than enough NK-EVs for pre-clinical experimentation or to meet dosing targets. Each isolation is tested for mycoplasma and microbial presence as part of the product's quality control assessment. In addition, a previous study demonstrated the absence of common viral entities and endotoxin in the final product and the absence of cellular components considered host cell contaminants (by western blot analysis)7,34. Lastly, functional assessment was performed using a validated highly sensitive resazurin-based cell viability assay to assess the NK-EVs' functionality20. The described viability assay functions by reducing resazurin (weakly fluorescent) to resorufin (highly fluorescent) by metabolically active cells, allowing for the assessment of cell viability following NK-EV treatment. Compared to other alternative cell viability assays, the resazurin-based assay used in the study is highly sensitive to changes in cell viability (very low background noise) and allows for shortened incubation time to observe results (less than 30 min to obtain statistically significant results)20. Generally, the NK-EVs exhibit a dose-dependent effect upon K562 viability. Together, the results presented represent an NK-EV product that has met the product release criteria for pre-clinical evaluation and is suitable for downstream applications.
In conclusion, this protocol-based study describes the biomanufacturing of NK-EVs with clinical-grade potential. As discussed, the NK-EVs are produced using a closed-loop HFB system under serum-free, xeno-free, feeder-free, and antibiotic-free conditions7. A combination of FPLC-SEC/UF isolates and purifies the NK-EV product. Before releasing the products for downstream application, the NK-EVs must be characterized and functionally validated to ensure they are suitable for use. As demonstrated, following this biomanufacturing protocol can successfully generate a large quantity of high-purity NK-EVs that exhibit on-target cytotoxicity against cancer cells. Therefore, the described biomanufacturing protocol may be an asset for future studies that require the production of clinical-grade NK-EVs.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge Drs. Simon Sauvé, Roger Tam and Xu Zhang for their critical manuscript review. This work was supported by operating grants from the Genomics Research and Development Initiative (GRDI) Phase VII (2019-2025) from the Government of Canada obtained by JRL, LW, as well as operating grants from the Natural Sciences and Engineering Research Council RGPIN-2019-05220, Cancer Research Society/University of Ottawa 24064, the Canadian Institutes of Health (CIHR) Research Operating Grant 175177 obtained by LW, the CIHR MSc Scholarship obtained by MK, and the Queen Elizabeth II Scholarships in Science and Technology (QEII-GSST) obtained by FSDB.
Materials
| 0.1 µm vacuum filtration unit Filtropur V50 | Sarstedt | 83,3941,002 | |
| 0.22 µm Acrodisc Syringe Filter | Pall Corporation | PN4612 | |
| 1 mL syringe | Thermo Fisher Scientific | MB9204560TF-LAB | |
| 10 kDa Centricon Plus-70 Centrifugal Filter | Sigma | UFC701008 | |
| 60 mL syringe | BD Biosciences | 309653 | |
| 96-well Flat Clear Bottom Black Polystyrene TC-treated Microplates | Costar | 3603 | |
| Agarose | Thermo Fisher Scientific | R0491 | |
| AKTA Fast Protein Liquid Chromatograph | GE Lifesciences | 29022094 | |
| BD PrecisionGlide Needle – 18G | BD Biosciences | 305196 | |
| Benzonase Nuclease | Sigma | E1014-25KU | |
| BioTek Synergy H1 Multimode Reader | BioTek | SH1M2G-SN | |
| Blue Juice Gel Loading Buffer | Invitrogen | 10816015 | |
| CaptoCore 700 resin | Cytiva | 17548102 | |
| Cellometer Auto 2000 Viability Counter | Nexcelom BioScience LLC | ||
| CryoStor CS10 freezing medium | Sigma | C2874 | |
| DPBS−/− | Fisher | BP399-1 | |
| Dual LED Blue/White Light Transilluminator | Invitrogen | LB0100 | |
| Duet P3202 Flow Control Pump | FiberCell Systems | ||
| Dulbecco's phosphate-buffered saline | Gibco | 14190250 | |
| Ethanol | Commercial Alcohols | P006EAAN | |
| Exosome-Depleted FBS | Gibco | A2720803 | |
| Fluorobrite DMEM | Gibco | A18967-01 | |
| Glucose meter | AccuCheck | Model 930 | |
| HiScale chromatography column 10/40 | Cytiva | 29360550 | |
| ImmunoCult-XF (GMP medium alternative) | StemCell Technologies | 100-0956 | |
| ImmunoCult-XF T Cell Expansion Medium | StemCell Technologies | 10981 | |
| Isopropyl Alcohol | EMD | PX1834-1 | |
| K562 cells | ATCC | CCL-243 | |
| LB media | BioBasic | SD7002 | |
| L-Lactate Assay Kit | Abcam | ab65331 | |
| Medium hollow-fibre cartridge | FiberCell Systems | C2011 | |
| MgCl2 | Sigma | M1028 | |
| Mycoplasma PCR detection kit | Abcam | ab289834 | |
| NanoSight NS300 | Malvern | ||
| NaOH | Supelco | SX0607N-6 | |
| NK92-MI cells | ATCC | CRL-2408 | |
| pH Strips-Mquant | Sigma | 1,09533 | |
| PrestoBlue HS Cell Viability Reagent Assay | Invitrogen | P50200 | |
| Qubit 4 Fluorometer | Invitrogen | ||
| Qubit dsDNA BR Assay Kit | Invitrogen | Q33262 | |
| Qubit dsDNA HS Assay Kit | Invitrogen | Q33231 | |
| Qubit Flex Assay Tube Strips | Invitrogen | Q33252 | |
| Qubit Flex Fluorometer | Invitrogen | Q33327 | |
| Qubit Protein BR Assay Kit | Invitrogen | A50669 | |
| Quick Load 1Kb Plus DNA ladder | NEB | N0469S | |
| SYBRSafe DNA Gel Stain Invitrogen | Invitrogen | S33102 | |
| Syringe pump | Harvard Apparatus | 984730 | |
| Triton-X 100 | Sigma | T-9284 | |
| UltraPure TAE Buffer | Invitrogen | 15558042 | |
| ViaStain Acridine Orange and Propidium Iodide (AO/PI) Staining Solution | ESBE Scientific | CS2-0106 |
Referanslar
- Cheng, M., Chen, Y., Xiao, W., Sun, R., Tian, Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 10 (3), 230-252 (2013).
- Sheridan, C. Industry appetite for natural killer cells intensifies. Nat Biotechnol. 41 (2), 159-161 (2023).
- Shimasaki, N., Coustan-Smith, E., Kamiya, T., Campana, D. Expanded and armed natural killer cells for cancer treatment. Cytotherapy. 18 (11), 1422-1434 (2016).
- Elsharkasy, O. M., et al. Extracellular vesicles as drug delivery systems: Why and how. Adv Drug Deliv Rev. 159, 332-343 (2020).
- St-Denis-Bissonnette, F., et al. Applications of extracellular vesicles in triple-negative breast cancer. Cancers. 14 (2), 451 (2022).
- Welsh, J. A., et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 13 (2), e12404 (2024).
- St-Denis-Bissonnette, F., et al. A clinically relevant large-scale biomanufacturing workflow to produce natural killer cells and natural killer cell-derived extracellular vesicles for cancer immunotherapy. J Extracell Vesicles. 12 (12), e12387 (2023).
- Federici, C., et al. Natural-killer-derived extracellular vesicles: Immune sensors and interactors. Front Immunol. 11, 262 (2020).
- Lugini, L., et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 189 (6), 2833-2842 (2012).
- Zhu, L., et al. Novel alternatives to extracellular vesicle-based immunotherapy – exosome mimetics derived from natural killer cells. Artif Cells Nanomed Biotechnol. 46 (sup3), S166-S179 (2018).
- Cochran, A. M., Kornbluth, J. Extracellular vesicles from the human natural killer cell line NK3.3 have broad and potent anti-tumor activity. Front Cell Dev Biol. 9, 698639 (2021).
- Kim, H. Y., et al. Delivery of human natural killer cell-derived exosomes for liver cancer therapy: an in vivo study in subcutaneous and orthotopic animal models. Drug Deliv. 29 (1), 2897-2911 (2022).
- Alvarez-Erviti, L., et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 29 (4), 341-345 (2011).
- El-Sahli, S., et al. A triple-drug nanotherapy to target breast cancer cells, cancer stem cells, and tumor vasculature. Cell Death Dis. 12 (1), 8 (2021).
- Sulaiman, A., et al. Co-targeting bulk tumor and CSCs in clinically translatable TNBC patient-derived xenografts via combination nanotherapy. Mol Cancer Ther. 18 (10), 1755-1764 (2019).
- Farcas, M., Inngjerdingen, M. Natural killer cell-derived extracellular vesicles in cancer therapy. Scand J Immunol. 92 (4), e12938 (2020).
- Murphy, D. E., et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 51, 1-12 (2019).
- Thery, C., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 7 (1), 1535750 (2018).
- FiberCell-Systems. . FiberCell systems user manual & quick start guide. , (2024).
- St-Denis-Bissonnette, F., et al. Evaluation of resazurin phenoxazine dye as a highly sensitive cell viability potency assay for natural killer cell-derived extracellular vesicle-based cancer biotherapeutics. J Extracell Biology. 3 (7), e166 (2024).
- Herrmann, I. K., Wood, M. J. A., Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 16 (7), 748-759 (2021).
- Andaloussi, E. L. A., Mager, I., Breakefield, X. O., Wood, M. J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 12 (5), 347-357 (2013).
- Federici, C., et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One. 9 (2), e88193 (2014).
- Yanez-Mo, M., et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 4, 27066 (2015).
- Neviani, P., et al. Natural killer-derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 79 (6), 1151-1164 (2019).
- Sun, H., et al. Natural killer cell-derived exosomal miR-3607-3p inhibits pancreatic cancer progression by targeting IL-26. Front Immunol. 10, 2819 (2019).
- Jiang, Y., et al. Engineered exosomes: a promising drug delivery strategy for brain disease. Curr Med Chem. 29 (17), 3111-3124 (2022).
- Dosil, S. G., et al. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. Elife. 11, e76319 (2022).
- Geeurickx, E., et al. The generation and use of recombinant extracellular vesicles as biological reference material. Nat Commun. 10 (1), 3288 (2019).
- Nathani, A., et al. Combined role of interleukin-15 stimulated natural killer cell-derived extracellular vesicles and carboplatin in osimertinib-resistant H1975 lung cancer cells with EGFR mutations. Pharmaceutics. 16 (1), 83 (2024).
- Gobin, J., et al. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res Ther. 12 (1), 127 (2021).
- Sun, L., et al. A 3D culture system improves the yield of MSCs-derived extracellular vesicles and enhances their therapeutic efficacy for heart repair. Biomed Pharmacother. 161, 114557 (2023).
- Ryu, A. H., Eckalbar, W. L., Kreimer, A., Yosef, N., Ahituv, N. Use antibiotics in cell culture with caution: genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci Rep. 7 (1), 7533 (2017).
- Meng, W., et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 27 (1), 585-598 (2020).
- Yang, Y., et al. Extracellular vesicles isolated by size-exclusion chromatography present suitability for RNomics analysis in plasma. J Transl Med. 19 (1), 104 (2021).
- Gamez-Valero, A., et al. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci Rep. 6, 33641 (2016).
- Gupta, D., Zickler, A. M., El Andaloussi, S. Dosing extracellular vesicles. Adv Drug Deliv Rev. 178, 178 (2021).
Etiketler

.
