Monitoring Blood-Brain Barrier Opening in Rats with a Preclinical Focused Ultrasound System
Özet
This study describes a combined magnetic resonance imaging (MRI) and low-intensity pulsed focused ultrasound (FUS) protocol, utilizing living rats with jugular vein catheterization to monitor blood-brain barrier (BBB) opening.
Abstract
The brain has a highly selective semipermeable blood barrier, termed the blood-brain barrier (BBB), which prevents the delivery of therapeutic macromolecular agents to the brain. The integration of MR-guided low-intensity pulsed focused ultrasound (FUS) with microbubble pre-injection is a promising technique for non-invasive and non-toxic BBB modulation. MRI can offer superior soft-tissue contrast and various quantitative assessments, such as vascular permeability, perfusion, and the spatial-temporal distribution of MRI contrast agents. Notably, contrast-enhanced MRI techniques with gadolinium-based MR contrast agents have been shown to be the gold standard for detecting BBB openings. This study outlines a comprehensive methodology involving MRI protocols and animal procedures for monitoring BBB opening in a rat model. The rat model provides the added benefit of jugular vein catheter utilization, which facilitates rapid medication administration. A stereotactic-guided preclinical FUS transducer facilitates the refinement and streamlining of animal procedures and MRI protocols. The resulting methods are characterized by reproducibility and simplicity, eliminating the need for specialized surgical expertise. This research endeavors to contribute to the optimization of preclinical procedures with rat models and encourage further investigation into the modulation of the BBB to enhance therapeutic interventions in neurological disorders.
Introduction
The BBB regulates the movement of ions, molecules, and cells between the blood vessels and brain tissues1. This function allows the maintenance of neuronal function and health while preventing the delivery of therapeutic macromolecular agents to the brain tissues. The limited permeability of the BBB can result in decreasing the efficacy and effectiveness of treatments for brain tumors and neurodegenerative diseases, such as Parkinson's disease, amyotrophic lateral sclerosis, and Alzheimer's disease2. Hence, various approaches, such as convection-enhanced delivery, direct intracranial injections, osmotic disruption, and enhanced drug design, have been investigated to bypass the BBB for controlled delivery of therapeutic agents3,4,5.
However, these approaches have intrinsic limitations such as invasiveness, risk of infection, risk of hemorrhage, heterogeneity, and rapid efflux of drugs. Low-intensity pulsed focused ultrasound (FUS) with the injection of microbubbles is a promising non-invasive technique allowing localized and reversible BBB disruption. The mechanical effects of ultrasound coupled with microbubbles can transiently alter the permeability of the BBB (i.e., BBB opening)6,7. For further advancements in treatment applications utilizing BBB opening, in vivo small-animal experiments are essential.
In this study, we introduced MRI protocols and animal procedures to monitor BBB opening in a rat model. The protocol included a rat model with a Jugular vein catheter cannulated by a commercial organization (see Table of Materials). A rat model of jugular vein catheterization has the potential benefits of rapid medication administration, hemodynamic monitoring tests, and stable catheterization. BBB opening was accomplished using a preclinical FUS system. Specifically designed for BBB opening in small animal models such as rats and mice, this system incorporates essential components, such as a benchtop system, control computer, transducer, water degasser, and accompanying accessories. The use of this stereotactic-guided preclinical FUS system not only enhances precision, but also streamlines animal procedures and MRI protocols, contributing to the reproducibility and simplicity of the overall experimental protocol.
Protocol
All animal experiments in this study were approved by the University of California, San Francisco (UCSF) Institutional Animal Care and Use Committee (IACUC). Male Sprague-Dawley rats (200-250 g) were used in this study. Rats were housed and cared for according to the National Institutes of Health Office of Animal Care and Use Guidelines in mice and rats. The details of the reagents and the equipment used are listed in the Table of Materials.
1. Animal preparation
NOTE: For in-house procedures, the animals were transported to the preparation room and the procedures were conducted as follows:
- Place the rats individually in a Lucite chamber and expose isoflurane (2%-3%) mixed with oxygen extracted from a medical oxygen tank until they are unconscious (following institutionally approved protocols).
NOTE: Anesthesia using isoflurane enables the implementation of a long-term MRI protocol. - Remove the rats from the chamber and shave hair from the anterior and posterior areas of the neck using a hair removal (depilatory) cream.
- Apply lubricant ointment to the eyes.
- Place the nose into the nose cone to maintain anesthesia with spontaneous respiration (isoflurane vaporizer between 2%-2.5%, oxygen flow meter to 1-1.5 L/min).
- Place the anesthetized rat onto a heated surgical table and confirm with a toe pinch.
NOTE: The MRI protocol included contrast-enhanced sequences, such as T1-weighted gradient echo imaging and T2-weighted spin echo imaging. These sequences were conducted both before and after injection of gadolinium (GAD)-based contrast agents to assess the blood-brain barrier (BBB) permeability. This involves administering GAD contrast agents while the rat is in an MRI scanner7. To facilitate this process, catheter tubing extending from the catheter cannulated in the rat was used. The extension included a catheter connector and polyethylene tubing measuring 30 cm. To ensure patency and prevent leakage, the extension tubing was flushed with heparinized glycerol (250 IU heparin/1 mL glycerol).
2. Stereotactic-guided focused ultrasound system
NOTE: The animals were transferred and placed on a table in a stereotactic FUS system. The rat's nose was placed into the nose cone to maintain anesthesia. The FUS system comprises a function generator, an RF amplifier, and an oscilloscope. Before setting up the transducer, we ensured the proper connection of all devices with the system. The transducer setup was completed using the following steps:
- Place a sharp pointer at the position of the Bregma in the skull and save the position.The position of Bregma can be identified by pointing the location of 9.0 mm anterior to Lambda on the midline8. The Lambda can be found at the center of the line between bilateral ear drums)9.
- Place ultrasound gel, water coupling bag, and the transducer (1 MHz frequency). Ensure that no air bubbles are present between the rat's skin, the water coupling bag, and the transducer.
- Connect a coaxial BNC cable of the transducer to the input of the FUS system module.
- In a control computer) Click on load for pre-registered rat images; a number of focal spots and acoustic pressure are chosen.
- In a control computer) Click on the motion test in the treatment moduleto ensure that the transducer can shift between spots within a burst period.
- Set up the sonication parameters described in step 3.
3. Microbubble infusion and sonication
- Activate the microbubble.
NOTE: The microbubble used in this study was characterized by a diameter of 1.7 µm and a concentration of 0.2 × 109 particles per milliliter10. The microbubble vial was activated based on the prescribing instructions provided by the vendor and a previously published report11.- Prepare a 5 mL syringe with a luer lock tip and filled with 5 mL of additive-free 0.9% sodium chloride injection.
- Remove the green cap, and connect the syringe to the vented dispensing pin (see Table of Materials) by screwing it clockwise.
- Remove the pin protection and place it in the center of the vial's rubber stopper. Press down firmly until the spike is fully inserted into the stopper.
- Empty the entire 5 mL syringe into the vial by pushing on the plunger rod.
- Shake the vial vigorously for 20 s to mix all the contents. A consistent white, milky liquid will show that sulfur hexafluoride lipid microspheres have formed.
- To obtain the required dose, invert the syringe, and slowly withdraw the intended volume of the suspension into a 5 mL syringe.
- Use an infusion pump.
NOTE: The infusion approach12 was used to deliver microbubbles to rats using a syringe pump. A syringe containing microbubbles was connected to a catheter connector inserted into the rat. Microbubbles were transferred through polyethylene tubing, and the infusion pump flow rate was set to 0.66 mL/min. This infusion rate allows the injection of 2 mL of microbubbles from a 3 mL syringe over 3 min. The infusion started before sonication for 1 min and continued throughout the 2 min sonication period. - Perform sonication.
NOTE: In this study, FUS pulses were chosen with the following parameters: (1) Target region: left hippocampus; (2) Number of spots: 6 to cover the hippocampus in the brain; (3) Burst length: 10 ms; (4) Burst period: 1200 ms; (5) Acoustic pressure: 0.65 MPa; (6) 'Burst mode' in the sonication mode menu in the FUS system; (7) Sonication time: 120 s.
4. MRI procedure using a 3T preclinical scanner
- After sonication, transfer the animals to a table in a preclinical 3T cryogen-free scanner.
- Position the rats in the prone position. Maintain the temperature of the heating pad in the scanner, and monitor the breathing rate using a pneumatic pillow sensor. Perform the MRI protocol to test various types of MR imaging (Table 1).
- Include other quantitative imaging techniques, such as diffusion-weighted imaging (DWI), T2* mapping, T1 mapping, and dynamic contrast-enhanced (DCE) MRI, in addition to contrast-enhanced MRI, to demonstrate its potential practicality for diverse imaging studies. Refer to Table 1 for the MRI protocols used in this study.
- Inject GAD-based MRI contrast agents (Gadavist, 1 mmol/kg) after the T1 weighted MR gradient echo sequence, and acquire post-GAD images.
NOTE: For a 200 g rat, we injected 200 µL of the MRI contrast agent and followed it with a 200 µL saline solution to flush the tubing.
5. Macroscopic examination of the rat brain
NOTE: After the MRI scan, 2 mL of Evans blue solution (2% diluted with phosphate-buffered saline) was injected, and the rat was allowed to rest for at least 30 min to allow the solution to spread and circulate throughout the body. Subsequently, the rats were euthanized using an overdose of isoflurane (5%) for macroscopic examination of the brain (following institutionally approved protocols). To confirm euthanasia, toe punching was required. The perfusion procedure was performed, followed by an examination of the rat brain. The perfusion procedure is summarized as follows:
- Perform a thoracotomy using a surgery scissor to expose organs in the chest and visualize the cardiac region.
- Incise (~5 mm) the right atrium.
- Introduce saline solution into the left ventricle for perfusion purposes.
- Open the skull bone with a surgical scissors to unveil and present the brain.
NOTE: Adequate perfusion should result in a uniformly bright appearance of the brain, while persisting blue areas indicate BBB-associated albumin leakage, as detected by Evans blue.
Representative Results
This study presents a preclinical protocol for monitoring BBB opening in a rat model using a commercial animal FUS system. The FUS system was set up on a bench or large cart carrier (Figure 1). Rats were anesthetized with isoflurane mixed with oxygen through the nose cone in the frame with the transducer holder. A hydrophone resonating at 500 kHz for detecting acoustic cavitation was inserted into the FUS transducer with a resonant frequency of 1.0 MHz.
As shown in Figure 2, the FUS system includes sonication settings and the capability to adjust target positions. A 'motion test' should be conducted to ensure that the transducer can translate between spots within a burst period.
When the BBB is opened, GAD and Evans blue molecules pass through the BBB. The T1-weighted MR image captured GAD-induced alterations in the MR signal intensity (Figure 3A). Following the perfusion procedure, the Evans blue-stained tissue was directly visible (Figure 3B).
Experiments with three rats were performed to demonstrate the reproducibility of BBBO. Figure 4A shows the T1-weighted gradient echo images after sonication and the GAD administration. A significant increase in the MR signal intensity was observed at the target across three experiments. DCE-MR images indicated that the most significant change in signal intensity occurred 7-8 min after the MRI contrast agent bolus (Figure 4B). T1-weighted gradient and T2-weighted spin echo sequences clearly showed a region of BBB opening, while no significant changes were observed in the T1 map before the GAD administration (Figure 4C).
The animal and MRI protocols proposed in this study demonstrate the feasibility of conducting MR imaging studies using a rat model with Jugular vein catheterization. MR perfusion imaging and/or diffusion tensor imaging can be integrated into the protocol to investigate a new MRI biomarker for detecting the BBB opening.
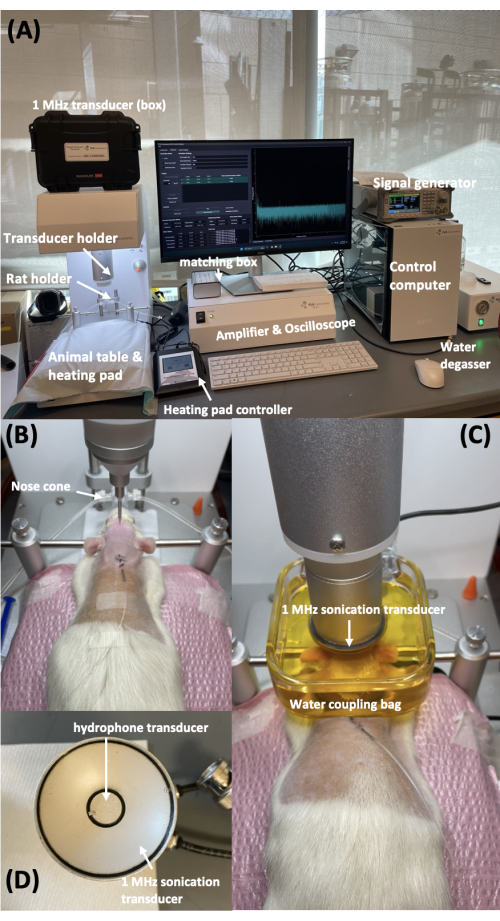
Figure 1: Benchtop preclinical FUS system and rat positioning. (A) Benchtop preclinical FUS system. (B) Rat positioning within the system. (C) 1 MHz transducer and hydrophone, with the hydrophone positioned at the center of the FUS transducer. (D) Positioning of the transducer and water coupling container on the rat's head. Please click here to view a larger version of this figure.
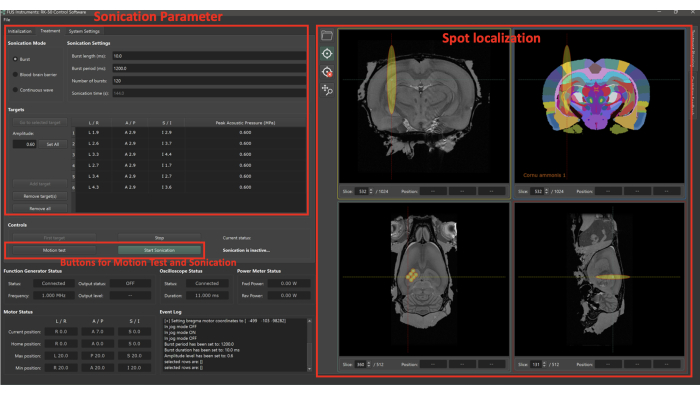
Figure 2: User interface of preclinical FUS system software. The user interface of the preclinical FUS system software allows modification of sonication settings and target positions. Before initiating sonication, the 'motion test' should be conducted to ensure that the transducer can shift between spots within a burst period. This function measures the movement time of the transducer across spots without sonication. Please click here to view a larger version of this figure.
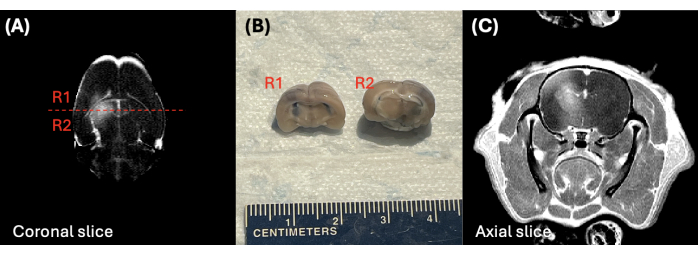
Figure 3: Post-GAD T1-weighted images and brain regions post-BBB opening. (A) T1-weighted post-GAD image and (B) photograph of the rat brain after BBB opening. The labels R1 and R2 in (A) correspond to the regions shown in (C). Please click here to view a larger version of this figure.

Figure 4: MR imaging post-sonication and GAD injection. (A) T1-weighted MR images of three rats after sonication and GAD injection. (B) Dynamic contrast-enhanced (DCE) MRI of Rat #3, illustrating time-variant changes in signal intensity. The GAD-based MRI contrast agent was injected at the time point indicated by the red arrow. (C) T1-weighted, T2-weighted MR images, and T1 maps of Rat #3, acquired prior to the injection of the GAD contrast agent. Please click here to view a larger version of this figure.
| 2D DWI | Cor 2D | Cor 2D T1 map | Cor 2D | Cor 2D | Cor 2D DCE (Bolus 1) | Cor 2D | Cor 2D | |
| R2* map | FLASH pre | RARE pre | FLASH post | RARE post | ||||
| Sequence | EPI | GRE | TSE | GRE | TSE | GRE | GRE | TSE |
| TR | 2100 ms | 500 ms | 2400, 1600, 1100, 800, 450, 300 ms | 30 ms | 2000 ms | 39.3 ms | 30 ms | 1500 ms |
| TE | 22.1 ms | 12 echos; | 10.29 ms | 5.1 ms | 65 ms | 2.9 ms | 5.1 ms | 65 ms |
| 3 – 47 ms | ||||||||
| Thickness | 1 mm | 1 mm | 1 mm | 1 mm | 1 mm | 1 mm | 1 mm | 1 mm |
| Field of view | 40 x 40 | 40 x 40 | 40 x 40 | 40 x 40 | 40 x 40 | 40 x 40 | 40 x 40 | 40 x 40 |
| Matrix | 192 | 192 | 192 | 256 | 192 | 128 | 256 | 192 |
| FA (degree) | 90 | 60 | 90/180 | 20 | 90/180 | 12 | 20 | 45/180 |
| Number of slices | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Acquisition time | 4 min 12 s | 3 min 12 s | 10 min 38 s | 2 min 9 s | 1 min 52 s | 8 min 23s | 3 min 52 s | 1 min 24 s |
| Options | Segment 12, 3 direction, b = 0, 54, 180, 522 s/mm2 | Number of Averages= 2, echo spacing= 4 ms | Echo spacing 10.3 ms, RARA factor 2 | RARE factor 10, Averages 4 | 200 µL agent (Gadavist, 1 mmol/kg) | RARE factor 10, Averages 4 | ||
| for 200 g rat, 1 min pre, 7 min post, Inject 200 µL/min, 200 µL saline flash |
Table 1: Preclinical MRI protocol at 3T MRI scanner.
Discussion
A protocol and animal procedure using a rat model of jugular vein catheterization for BBB opening studies has been developed. While numerous preclinical protocols exist, this protocol incorporates a commercially available FUS system and a rat model, minimizing animal preparation procedures and thereby ensuring experimental reproducibility.
In this protocol, microbubble selection was based on its stability and room temperature storage. Other commercial microbubbles may be considered as alternatives. Kotopoulis et al. investigated and compared various microbubble types for low-intensity sonoporation of pancreatic cancers10. Based on in vitro results, the selected microbubble in this study was found to be the optimal formulation at lower intensities, while other microbubbles were more effective at higher intensities. However, the most suitable microbubbles for opening the BBB in a rat model remain unclear. Vlachos et al.13 investigated the effects of acoustic pressure and microbubble size on the volume of BBB opening and tissue damage, such as vascular disruption and edema. The suggested protocol encourages future studies to investigate and identify the most suitable microbubble and sonication parameters for use in a rat model.
In this study, the preclinical FUS system was selected due to its rapid and precise capabilities for multi-point targeting. The system is programmed to automatically traverse pre-defined focal points, a feature that facilitates regional sonication directed at the targeted tissue area. As an alternative, established focused ultrasound (FUS) transducers, such as those offered by Sonic Concepts and ExAblate Neuro (see Table of Materials), may be employed to open the blood-brain barrier14,15. Notably, the ExAblate Neuro transducer is designed to be compatible with MR imaging, providing real-time guidance and monitoring of the treatment process. In the future, these transducers can be integrated into the developed protocol.
The proposed MRI protocol incorporates various MRI sequences to demonstrate its potential practicality for diverse imaging studies. Pre- and post-contrast T1-weighted gradient echo sequences and T2-weighted spin echo sequences are recommended to capture changes in signal intensity induced by MRI contrast agents from BBB leakage. Users have the option to include DWI, DCE, and T2* mapping in their imaging protocol. As an alternative to using an MRI scanner, Singh et al.16 introduced methods using Doppler ultrasound imaging for focal localization and passive acoustic mapping to detect the cavitation of microbubbles, which is correlated with BBB-opened regions. Therefore, this protocol holds promise for implementation within an all-ultrasound system, presenting a potential alternative.
In this study, the infusion approach12 was employed, which refers to infusion during sonication. Since this approach continuously provides microbubbles to the brain, it may result in a more stable and consistent opening of the BBB compared to bolus infusion. Lapin et al.12 investigated the comparison between the infusion and bolus approaches for consistent BBB opening, finding that a rate of 7.2 µL Definity microbubble/kg/min or below could open the BBB without injury at various pressure levels. However, this approach may have a potential limitation in maintaining the concentration of microbubbles within the syringe over time. Microbubbles may tend to float and aggregate at the top of the syringe, possibly causing a decrease in the microbubble dose. Alternatively, bolus injection of microbubbles can be conducted into the retroorbital sinus. Future studies using the proposed protocol should explore comparisons with different microbubble infusion methods and types to optimize BBB opening in a rat model.
The limitation of this study is the absence of histochemical validation for macroscopic damage, such as Hematoxylin and Eosin (H&E) or Crystal Violet staining results. However, the proposed protocol can be utilized to optimize sonication and MRI parameters to avoid macroscopic damage in the brain. Additionally, procedures for histopathology analysis can be incorporated in future studies to address this limitation.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This study was partially supported by the National Institute of Dental and Craniofacial Research (NIDCR) Grant K99DE032397, UCSF Department Seed Grant (No. 7504831), UCSF RAP grant (PI: Kazim Narsinh, MD), and the German Research Foundation (DFG, GA 3535/1-1).
Materials
| 1 mL syringe | BD plastipak | ||
| 20 mL, Sodium Chloride 0.9% | Hospira, Inc | 00409-7101-67 | |
| 3 mL syringe | BD plastipak | ||
| 5 mL syringe | BD plastipak | ||
| Blunt Needle, 21 G | SAI infusion technologies | B21-50 | |
| Catheter connector, 21 G | SAI infusion technologies | CC-25-25 | |
| Catheter lock solution | SAI infusion technologies | Sku # HGS-10 | (500 µ/mL) Glycerol |
| Evans Blue | Sigma aldrich | E2129-10g | |
| ExAblate Neuro | Insightec Inc., Israel | ||
| Gadavist | Bayer | 2068062 | |
| GE isoflurane Vaporizer | GE Healthcare | ||
| IPS-12RS Syringe Pump | Inovenso | IPS-12 | |
| isoflurane USP | Vet one | 501017 | |
| Lumason microbbuble | Bracco Diagnostics Inc | SKJ709700 | |
| Male Sprague-Dawley rats (200–250 g) | Charles River Laboratories | ||
| Needle, 18 G | BD Precisionglide | ||
| Phosphate-buffered saline | Mediatech | 21-030-CVR | |
| Polyethylene Tubing | BD Intramedic | ||
| preclinical 3T cryogen-free Bruker Biospin scanner | Bruker | Biospec3T | |
| Rat with jugular vein catheterization | Charles River Laboratories | ||
| RK-50 focused ultrasound transducer | FUS Instruments | Tx-50-1000-0 | |
| RK-50 benchtop focused ultrasound system | FUS Instruments | ||
| Sonic Concepts | Sonic Concepts Inc., WA, USA | ||
| vented dispensing pin | B Braun | 4550560 |
Referanslar
- Daneman, R., Prat, A. The Blood-Brain Barrier. Cold Spring Harb Perspect Biol. 7 (1), a020412 (2015).
- Meng, Y., et al. Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol. 17 (1), 7-22 (2021).
- Mungur, R., et al. Low-intensity focused ultrasound technique in glioblastoma multiforme treatment. Frontiers in Oncology. 12, 903059 (2022).
- Boado, R. J., et al. Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol Pharm. 7 (1), 237-244 (2010).
- Hendricks, B. K., et al. Novel delivery methods bypassing the blood-brain and blood-tumor barriers. Neurosurgical Focus. 38 (3), E10 (2015).
- Hynynen, K., et al. Non-invasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 220 (3), 640-646 (2001).
- Hynynen, K., et al. Local and reversible blood-brain barrier disruption by non-invasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage. 24 (1), 12-20 (2005).
- Yao, X., et al. Non-invasive, targeted creation of neuromyelitis optica pathology in AQP4-IgG seropositive rats by pulsed focused ultrasound. J Neuropathol Exp Neurol. 78 (1), 47-56 (2019).
- Cecyn, M. N., Abrahao, K. P. Where do you measure the Bregma for rodent stereotaxic surgery. IBRO Neurosci Rep. 15, 143-148 (2023).
- Kotopoulis, S., et al. SonoVue vs. Sonazoid vs. Optison: Which bubble is best for low-intensity sonoporation of pancreatic ductal adenocarcinoma. Pharmaceutics. 14 (1), 98 (2022).
- Bracco Diagnostics Inc. . LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use. , (2016).
- Lapin, N. A., et al. Consistent opening of the blood brain barrier using focused ultrasound with constant intravenous infusion of microbubble agent. Sci Rep. 10, 16546 (2020).
- Vlachos, F., et al. Permeability dependence study of the focused ultrasound-induced blood-brain barrier opening at distinct pressures and microbubble diameters using DCE-MRI. Magn Reson Med. 66 (3), 821-830 (2011).
- Singh, A., et al. An all-ultrasound cranial imaging method to establish the relationship between cranial FUS incidence angle and transcranial attenuation in non-human primates in 3D. Sci Rep. 14 (1), 1488 (2024).
- Plaksin, M., et al. Magnetic resonance imaging analysis predicts nanoparticle concentration delivered to the brain parenchyma. Commun Biol. 5 (1), 1-11 (2022).
- Singh, A., et al. Guiding and monitoring focused ultrasound mediated blood-brain barrier opening in rats using power Doppler imaging and passive acoustic mapping. Sci Rep. 12 (1), 14758 (2022).
Etiketler

.
