Social Isolation Model: A Noninvasive Rodent Model of Stress and Anxiety
Özet
Presented here is a social isolation (SI)-induced anxiety mouse model that utilizes wild type C56BL/6J mice to induce stress and anxiety-like behavior with minimal handling and no invasive procedures. This model reflects modern life patterns of social isolation and is ideal for studying anxiety and related disorders.
Abstract
Anxiety disorders are one of the leading causes of disability in the United States (US). Current treatments are not always effective and less than 50% of patients achieve full remission. A critical step in developing a novel anxiolytic is to develop and utilize an animal model, such as mice, to study pathological changes and test drug target(s), efficacy, and safety. Current approaches include genetic manipulation, chronic administration of anxiety-inducing molecules, or the administration of environmental stress. These methods, however, may not realistically reflect anxiety induced throughout daily life. This protocol describes a novel anxiety model, which mimics the intentional or unintentional patterns of social isolation in modern life. The social isolation-induced anxiety model minimizes perceived distractions and invasiveness and utilizes wild type C57BL/6 mice. In this protocol, 6- to 8-week-old mice (male and female) are singly housed in opaque cages to visually block the external environment, such as neighboring mice, for 4 weeks. No environmental enrichments (such as toys) are provided, bedding material is reduced by 50%, any treatment of drug is administered as an agar form, and the exposure/handling of the mice is minimized. Socially isolated mice generated using this protocol exhibit greater anxiety-like behavior, aggression, as well as decreased cognition.
Introduction
Anxiety disorders represent the largest class and burden of mental diseases in the United States (US), with related annual costs exceeding US$42 billion1,2,3. In recent years, anxiety and stress have heightened the prevalence of suicide and suicide ideation by over 16%4. Patients with chronic diseases are especially vulnerable to unintended secondary effects of mental distress or reduced cognitive function5. Current treatments for anxiety include psychotherapy, medications, or a combination of both6. However, despite this crisis, less than 50% of patients achieve full remission6,7. Anxiolytics such as benzodiazepines (BZs) and selective serotonin reuptake inhibitors (SSRIs) have significant drawbacks or produce little to no immediate effects8. Moreover, there is a relative scarcity of novel anxiolytics under development, challenged by the costly and time-consuming process of drug development9,10.
A critical step in the drug development process is the establishment and utilization of an animal model, such as mice, to study pathological changes and test drug safety and efficacy11. Current approaches to establishing anxiety animal models include 1) genetic manipulation, such as knocking out serotonin receptors (5-HT1A) or γ-aminobutyric acid A receptor (GABAAR) α subunits12; 2) chronically administering anxiety-inducers such as corticosterone or lipopolysaccharides (LPS)13,14; or 3) administering environmental stress including social defeat and maternal separation15. These methods, however, may not realistically reflect anxiety induced throughout daily life and therefore may not be suitable for investigating the underlying mechanism or testing novel drugs.
Like humans, mice and rats are highly social creatures16,17,18. Social contact and social interactions are essential for optimal brain health and are critical for proper neurodevelopment during the rearing period19. Thus, maternal separation or social isolation during the rearing period results in mice that show more anxiety, depression, and changes in neurotransmission20. Moreover, social grooming or allogrooming is a common form of bonding or comforting behavior among mice and rats that live together21. Thus, socialization is an integral part of rodent life, and isolation negatively impacts their health.
In this context, the present protocol describes a novel anxiety model to mimic the intentional or unintentional patterns of social isolation in modern life. This social isolation (SI) model minimizes perceived distractions and invasiveness and utilizes adult wild type C57BL/6 mice and Sprague-Dawley (SD) rats. The protocol presented here focuses on the anxiety mice model based on our published evidence, which showed increased anxiety-like behavior, aggression, decreased cognition, and increased neuroinflammation as a result of social isolation22,23,24. Anxiety-like behavior is confirmed by the elevated plus maze (EPM) and open field (OF) tests, while cognitive function is measured by novel object recognition (NOR) and novel context recognition (NCR) tests. This model is useful for investigating anxiety and related disorders but can also be adapted or modified to study the natural progression and development of mild cognitive impairment as well as metabolic changes due to stress.
Protocol
All animal experiments are performed according to the protocols approved by the University of Southern California (USC) Institutional Animal Care and Use Committee (IACUC), and all methods are carried out in accordance with relevant guidelines, regulations, and recommendations.
1. Animals
- Obtain approval from appropriate animal care committees for the study.
- Set the vivarium to a dark-light 12 h cycle with controlled temperature and humidity between 24 ± 2 °C and 50%-60%, respectively.
- Obtain male and/or female wild type C57BL/6 mice aged 6-8 weeks. After stratifying the animals by sex, randomly assign them to one of the following groups: 1) group house with vehicle treatment; 2) group house with drug treatment; 3) social isolation with vehicle treatment; or 4) social isolation with drug treatment. Aim for at least four mice per group per sex (ideally six mice per group).
- Upon arrival of the mice, acclimate them to the vivarium for at least 24 h. The mice should arrive singly housed.
2. Cage setup
- For social isolation animals, take a standard mouse cage (75 in2 floor space) and add half the amount of bedding and a 1 in2 piece of cotton (or equivalent) for nesting.
- Wrap the outside walls of the cages in opaque, black plastic bags (or equivalent) and secure using tape. Make sure the mice cannot see the outside environment or surrounding animals.
- Leave the top and bottom of the cage unwrapped, unless the mice can see neighboring animals through them.
- When wrapping, ensure that no segment of the bag is accessible from the inside of the cage. This is to prevent the animal from tearing the bag apart.
- Do not include any form of environmental enrichment, such as toys or running wheels.
- Carefully and gently place the mice in the prepared cages. Provide food and water ad libitum.
- House control mice in groups of two or three under normal caging conditions (i.e., in a standard mouse cage [75 in2 floor space], a full amount of bedding, a 2 in2 piece of cotton or equivalent for nesting, and no wrapping of opaque bags).
- Ensure the group-housed mice are compatible with each other (i.e., there are no fighting/conflicts between them). If conflict occurs, remove the aggressor and exclude from the analysis.
- Separate male and female mice housing and keep distance between the males and females to avoid the possibility of affecting the endocrine level changes of female mice due to their ability to smell.
3. Care and treatment during the social isolation period
- Disturb the mice as minimally as possible during the social isolation period. Perform any procedures and activities, such as cage changes and treatment administration, during their active period (i.e., during the dark cycle) and under minimal noise disturbances.
- Change the cages only once a week during the dark cycle. The same plastic bag can be removed and rewrapped to new cages, unless significant damage is present.
- For control (group housed) mice, change the cages twice a week or more as necessary during the dark cycle.
- Ensure the mice have plenty of water and food to last at least 1 week.
- Continue isolating (or group housing) the mice for at least 4 weeks to see optimal results.
4. Agar drug/treatment preparation-a noninvasive drug treatment
- If treatments (e.g., drugs under investigation) are involved in the study, ideally administer the treatment with as little handling as possible, by utilizing agar forms. Routes such as injection and oral gavage inflict additional stress on the mice that may become a confounding factor of anxiety.
- Adjust the timing and frequency of treatment based on the nature of the drug used.
NOTE: In this study, 2 mg/kg dihydromyricetin (DHM, [(2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one]) was used as the treatment. DHM was administered daily, in a single dose, during the dark phase of the last 2 weeks of the isolation (or group house) period. - To prepare the treatment, add 3% (w/v) agar in deionized (DI) water and heat to ~90 °C to dissolve. The solution will bubble. Prevent spillage or boiling over.
NOTE: Heat the solution in a glass flask via short, 10 s intervals of microwaving.
CAUTION: The glassware will be hot. Wear appropriate personal protective equipment (PPE) when handling the solution. - Swirl the solution and visually ensure a homogenous solution.
NOTE: The solution should be translucent and light yellow to light brown in coloration. - While the solution is still warm, add 5% (w/v) sucrose and the desired dose of treatment. Only add sucrose and do not add the treatment of interest to the vehicle control.
- Swirl the solution and visually ensure a homogenous solution. Then, pour the solution into a mold and let cool at room temperature to solidify. If the treatment is light-sensitive, make sure to protect it from light.
NOTE: The solution should be slightly viscous. - Once solidified, cut the agar into cubes of 0.5 cm x 0.5 cm x 0.5 cm and store at 4 °C until administration.
- To administer the treatment, place a single cube onto a small weigh boat. During the dark phase of the light-dark cycle, quietly and carefully place the agar-weigh boat into individual cages, without touching the mouse. Allow the mouse to consume the agar.
NOTE: Mice typically spend 15-45 min to completely consume the agar. - Confirm complete consumption of the agar and then carefully remove the weigh boat from the cage. Repeat as necessary.
- Prepare agar cubes weekly to keep fresh and avoid any contamination.
5. Behavior analysis
- Perform behavioral tests 24 h after the last day of the 4 week (or more) isolation period. Conduct tests during the dark phase under indirect red lighting and record with a video camera.
- Arrange at least three individuals to conduct manual offline scoring in a double-blinded manner to minimize bias and error.
- Elevated plus maze (EPM)
- Prepare the EPM apparatus. The apparatus used in this protocol was obtained commercially (see Table of Materials) and made of opaque plastic with two open arms and two closed arms (33 cm x 5 cm each, open arms perpendicular to the closed arms) with a center platform of 5 cm x 5 cm. Elevate the apparatus 50 cm above the floor.
- Place the animal on the center of the apparatus, facing an open arm. Allow the animal to explore for 5 min and record their activity using a video camera.
- Clean the apparatus after each animal by thoroughly wiping all surfaces with disinfectant (70% ethyl alcohol). Ensure that all rodent droppings are wiped off.
- Score the mice's behavior offline based on time spent in open arms, closed arms, and the center platform using a stopwatch. Start the stopwatch when the mouse places at least three paws in the respective arm or platform.
- Open field (OF) test
- Prepare the OF apparatus. The apparatus used in this protocol (see Table of Materials) was made of opaque plastic measuring 50 cm x 50 cm x 38 cm (length x width x height).
- Draw square grids (10 cm x 10 cm each) on the field for a total of 25 grids.
- Place the animal on the center of the field and allow to explore for 10 min. Record their activity on a video camera.
- Clean the apparatus after each animal by thoroughly wiping all surface with disinfectant (70% ethyl alcohol). Ensure that all rodent droppings are wiped off.
- Score the mice's behavior offline based on the time spent in the central zone, time spent in the corners, total distance traveled, and number of times the mouse reared.
- Use a stopwatch to record the time spent in the center or corner. Start the stopwatch when the mouse places at least three paws in the respective area.
- Use a counter to record the distance traveled and frequency of rearing. Count the number of squares the mouse enters (when the mouse places at least three paws in the square). Count rearing when the mouse clearly stands up on its hind paws. Do not count when the mouse stands up and leans against the walls or when it stands up to groom.
- Novel object recognition (NOR) test
- Perform this test over 3 days. On day 1, prepare an open field apparatus of 50 cm x 50 cm x 38 cm (length x width x height). Place the animal in the center of the open field and allow to familiarize for 5 min. Then place the animal back in its home cage.
- Clean the apparatus after each animal by thoroughly wiping all surfaces with disinfectant (70% ethyl alcohol). Ensure that all rodent droppings are wiped off.
- On day 2, prepare the same open field apparatus and place two identical objects, such as a small cube. Place them symmetrically about 20 cm apart. Place the animal in the center of the apparatus and allow to explore for 5 min. Then place the animal back in its home cage.
- Clean the apparatus after each animal by thoroughly wiping all surfaces with disinfectant (70% ethyl alcohol). Ensure that all rodent droppings are wiped off.
- On day 3, prepare the same open field apparatus and one of the objects from day 2 (i.e., small cube), which will function as the familiar object. Place another, dissimilar novel object, such as a wooden pyramid, symmetrically from the familiar object about 20 cm apart. Allow the animal to explore for 3 min and record their activity on a video camera.
- Clean the apparatus after each animal by thoroughly wiping all surfaces with disinfectant (70% ethyl alcohol). Ensure that all rodent droppings are wiped off.
- Score the mice's behavior offline based on the time spent exploring the familiar object and the novel object. Calculate the object recognition index (ORI%), where
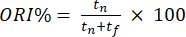 ; tf and tn represent the times of exploring the familiar and novel objects, respectively.
; tf and tn represent the times of exploring the familiar and novel objects, respectively.
- Perform this test over 3 days. On day 1, prepare an open field apparatus of 50 cm x 50 cm x 38 cm (length x width x height). Place the animal in the center of the open field and allow to familiarize for 5 min. Then place the animal back in its home cage.
- Novel context recognition (NCR) test
- Perform this test over 2 days. Prepare two distinctly shaped open fields and two pairs of distinctly shaped objects. The OF apparatus can be used as one of the contexts (open field). The other context should be of similar size but different shape, such as a round open field.
- On day 1, place one pair of identical objects (i.e., two cubes) in the square context and the other pair of identical objects (i.e., two pyramids) in the round context. Objects should be placed symmetrically 15-20 cm apart.
- Place the animal in the center and allow to explore for 5 min in one context. Repeat in the other context. Then, place the animal back in its home cage.
- Clean the apparatus after each animal by thoroughly wiping all surfaces with disinfectant (70% ethyl alcohol). Ensure that all rodent droppings are wiped off.
- On day 2, swap one of the objects from one context with the other (i.e., place one cube and one pyramid in the square context, and one cube and one pyramid in the round context).
- Place the animal in the center and allow to explore for 3 min. Record their activity on a video camera. The animals do not need to be recorded in both contexts.
- Clean the apparatus after each animal by thoroughly wiping all surfaces with disinfectant (70% ethyl alcohol). Ensure that all rodent droppings are wiped off.
- Score the mice's behavior offline based on the time spent exploring the distinct objects. Calculate the recognition index (RI%) as the proportion of time spent investigating the novel "out-of-context" object (i.e., the pyramid in the square context) versus the familiar "in-context" object (i.e., the cube in the square context).
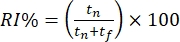 .
.
Representative Results
All representative results and figures were modified from our recent publications22,23. To evaluate the effects of social isolation on anxiety and exploratory behavior, EPM and OF tests were performed 24 h following the end date of the 4 week social isolation period. Socially isolated mice spent significantly less time in the open arm (1.28 ± 0.17 min) compared to the control (2.31 ± 0.27 min), and a significantly longer time in the closed arm (3.31 ± 0.27 min) compared to the control (2.24 ± 0.31 min) (Figure 1). Likewise, in the OF test, socially isolated mice traveled less (2,176 ± 146 cm vs. control [2,765 ± 161 cm]), reared less (28.25 ± 2.07 vs. control [46.63 ± 1.52]), spent more time in the corners (73.00 ± 4.31 s vs. control [28.25 ± 2.07 s]), and spent less time in the central area (7.63 ± 0.86 s vs. control [19.63 ± 0.71 s]), indicating enhanced anxiety-like behavior (Figure 2).
Furthermore, the effects of social isolation on cognition were assessed, as anxiety disorders typically also show symptoms of cognitive impairment, such as memory loss and difficulty concentrating25,26. Two tests were utilized: novel object recognition (NOR) and novel context recognition (NCR), as described earlier23, to assess the mice's ability to recognize novel objects under similar context (NOR) and novel context with similar objects (NCR). Socially isolated mice displayed both reduced novel object recognition (55.3 ± 4.1% vs. control [66.3 ± 4.7%]) (Figure 3A) as well as reduced novel context recognition (51.5 ± 6.5% vs. control [68.6 ± 2.8%]), suggesting cognitive impairment (Figure 3B).
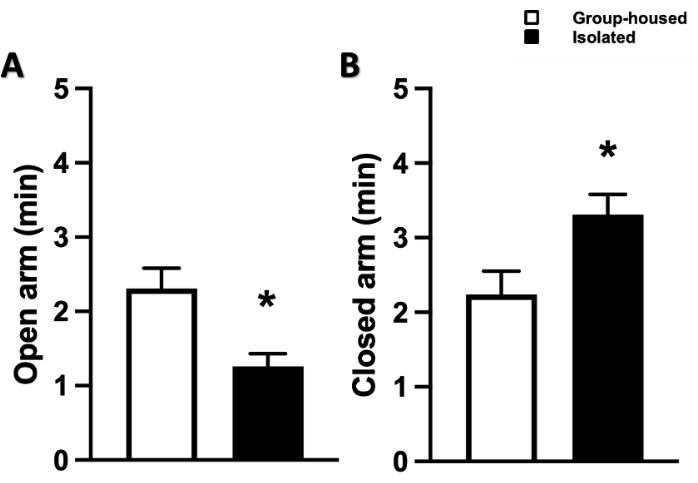
Figure 1: Changes in anxiety-like behavior as measured by the elevated plus maze (EPM). Time spent in the (A) open arm and (B) closed arm of the EPM apparatus. Data represented as mean ± SEM. One-way ANOVA followed by multiple comparisons, Holm-Sidak method. N = 11 per group. * p ≤ 0.05. This figure has been modified from Al Omran et al.22 (open access under a Creative Commons Attribution 4.0 International License). Please click here to view a larger version of this figure.
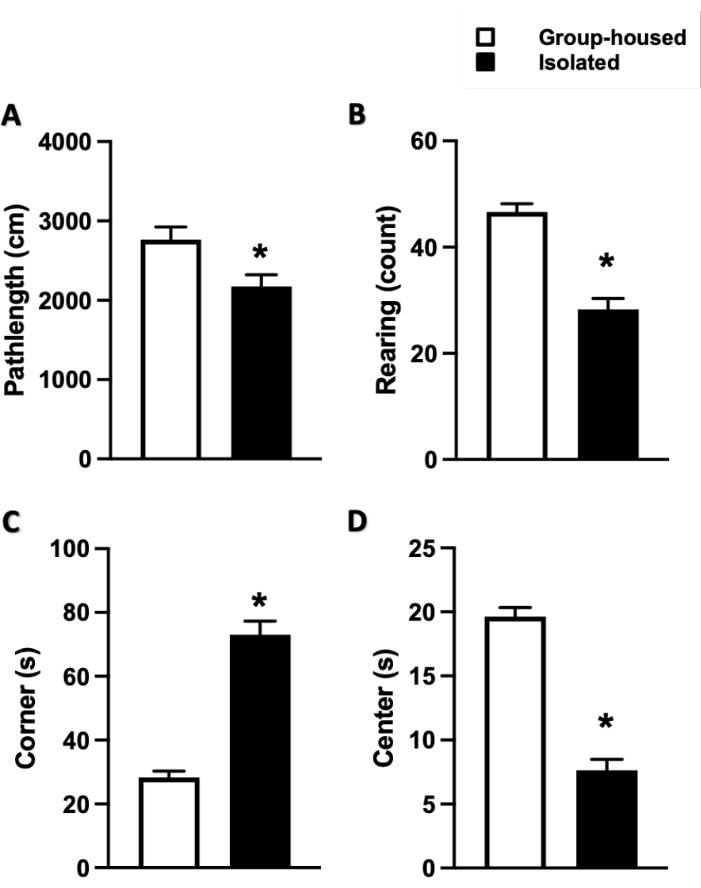
Figure 2: Changes in anxiety-like behavior and locomotor activity measured by the open field (OF) test. Data shown as (A) total distance traveled, (B) number of times the mice reared, (C) total time spent in the corner, and (D) total time spent in the center of the OF apparatus. Data represented as mean ± SEM. One-way ANOVA followed by multiple comparisons, Holm-Sidak method. N = 11 per group. * p≤ 0.05. This figure has been modified from Al Omran et al.22 (open access under a Creative Commons Attribution 4.0 International License). Please click here to view a larger version of this figure.
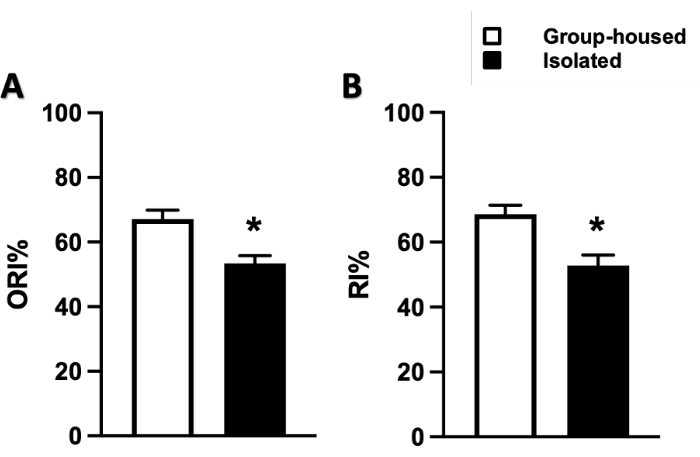
Figure 3: Changes in cognition as measured by novel object recognition (NOR) and novel context recognition (NCR) tests. (A) ORI = object recognition index. (B) RI = (novel context) recognition index. Data represented as mean ± SEM. One-way ANOVA followed by multiple comparisons, Holm-Sidak method. N = 9 per group. * p ≤ 0.05. This figure has been modified from Watanabe et al.23 (open access under a Creative Commons Attribution 4.0 International License). Please click here to view a larger version of this figure.
Discussion
Critical steps in the protocol include properly setting up the social isolation cages (i.e., wrapping of opaque bags and reducing the amount of bedding), minimizing the handling and disturbance of mice throughout the isolation period, and making sure the mice obtain and consume the agar with drug completely. It is critical that the vivarium or housing condition is maintained at a constant temperature and humidity, as well as minimized external interferences. Significant effort should be placed toward reducing as much of these confounding factors as possible, including but not limited to noise disturbance (e.g., conversing, equipment noises, etc.), overhandling, and disturbance of the animals during the light phase of the dark-light cycle. The time it takes to change cages, refill food and/or water, provide treatment, and all other functions during the isolation period should be minimized as well. Though rare, we have observed fighting among group-housed male mice in the past. Thus, for the group-housed mice (control or likewise), careful observation is needed to ensure that there are no conflicts among the mice, as this would factor in as another confounder of anxiety or stress. In the case that fighting does occur, the suspected aggressor should be swapped with another group-housed counterpart and continue to be observed. If the aggressor continues to create conflicts, it is suggested to exclude the aggressor as well as mice that have suffered injuries from the aggressor from the study.
The described protocol recommends 4 weeks of isolation, but this timeframe may be increased. The longest period of social isolation that we have performed is 8 weeks, and we have also performed repeated social isolation (isolation, group house, re-isolation) as a model of accumulated anxiety/stress. The timing and length of these isolation periods may be modified to fit experimental needs or purposes. However, decreasing the isolation period to less than 4 weeks is not recommended, as it may not be enough time for the mice to exhibit anxiety-like behavior or brain pathological changes. The timing and frequency of treatment(s) may be modified as well.
With respect to existing methods for establishing anxiety animal models, this model has several advantages. First, it does not require extensive phenotype selection (selective breeding) or genetic manipulation, such as knocking out or silencing receptors in the brain. While genetically modified mice are useful for investigating susceptible genes, they may not wholly capture anxiety pathogenesis12. Further, gene knockouts could be lethal or fail to accurately mimic anxiety observed in humans27. Genetic manipulation takes time and effort, requiring embryonic stem cell extraction, DNA injection, culturing, implantation into the uterus, and rearing27. In addition, these genetic animals may not truly reflect drug effects for development of drugs. This social isolation model, although requiring at least 4 weeks of isolation, is advantageous in terms of time, effort, and reliability. Second, the mice do not have to be chronically administered with anxiety-inducers such as corticosterone or lipopolysaccharides (LPS)13,14. There is no need for investigators to go through daily injection procedures, and the social isolation model more accurately reflects anxiety in humans, as most individuals do not receive daily injections to experience anxiety. Lastly, mice do not need to be conditioned (such as in social defeat paradigms), which takes time and may not generate reproducible levels of anxiety (i.e., significant variation among mice)15.
Many of the currently available social isolation models begin the isolation period in early development, between neonatal to juvenile and adolescent periods. Such early-life isolation models induce depressive- and anxiety-like behaviors, social avoidance behaviors, and other neuropsychiatric symptoms that mirror anxiety disorders, depression, autism, and related mental disorders28. While the early-life social isolation method is well-established and commonly used, it does not wholly reflect the development of mental disorders, as not all individuals experience maternal separation (social isolation) during their adolescent years29. Moreover, their effects vary based on species, strain, sex, and frequency/duration of the isolation28. For example, some studies have found post-wean social isolation to increase aggressive behavior in C57BL/6J mice, while others have shown only a small or no effect28. This variation is likely due to slight differences in the frequency, duration, or housing setup of the isolation period. Another study with mice in the adult or late-life stage found social isolation to increase hyperactivity, with no apparent depressive- or anxiety-like behaviors30. These mice were unable to see neighboring mice, similar to our model, but utilized female F1 hybrid C57BL/6J x 129S6/SvEvTac mice30, suggesting the variability among strains and sex. This study hopes to minimize these variations by proposing a consistent method.
A drawback to this technique is that the sound factor is not eliminated. Because the cages are not soundproof, animals are still able to hear each other, and thus may not be in absolute isolation. It may be of interest to incorporate a soundproof cage into the protocol and investigate the effects of auditory isolation on anxiety and cognition. For the purposes of this model, however, only visual senses and interactions are blocked as this model is not a sensory deprivation model, but rather a model to prohibit face-to-face social interaction. The model intends to mimic the in-person social interactions, as auditory stimulus is typically present in human life. Another drawback is that this protocol has only been tested in C57BL/6 mice and Sprague Dawley rats. As mentioned earlier, effects of social isolation can vary based on species and strain. Although the reproducibility of this protocol in other rodent species/strains cannot be guaranteed, it can be confirmed that this model can be consistently recreated in these two animals.
As the animals exhibited reduced cognition and memory, this model may be developed as a mild cognitive impairment model. Though further optimization is needed, the model may be useful for investigating the mechanism of social isolation-induced cognitive impairment, perhaps from accumulated episodes of stress and anxiety. The model may also be used to study the effect of later-life social isolation on social behaviors, aggression, or violence.
Overall, the social isolation-induced anxiety mouse model can be applied to investigating anxiety and related disorders in a noninvasive, minimally handled manner, and aims to accurately mimic anxiety induced from social isolation and loneliness.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This work was funded by the National Institute of Health grant AA17991 (to J.L.), Carefree Biotechnology Foundation (to J.L.), University of Southern California (USC), USC Graduate School Travel/Research Award (to S.W.) Saudi Arabia Cultural Mission Scholarship (to A.A.O.), and Army Health Professions Scholarship Program (to A.S.S.).
Materials
| Black Plastic Bags | Office Depot | 791932 | 24" x 32" |
| Elevated Plus Maze | SD Instruments | NA | Black color |
| Open Field enclosure | SD Instruments | NA | White color |
| Select Agar | Invitrogen | 30391-023 | |
| Square cotton for nesting (nestlet) | Ancare Corporation | NC9365966 | Divide a 2" square piece into 4 pieces to create a 1" square piece for isolation group |
| Sucrose | Sigma | S1888-1KG | |
| Weigh boat | SIgma | HS1420A | Small, square white polystyrene |
Referanslar
- Craske, M. G., et al. Anxiety disorders. Nature Reviews Disease Primers. 3 (1), 17024 (2017).
- Kasper, S., den Boer, J., Ad Sitsen, J. . Handbook of Depression and Anxiety: A Biological Approach. , (2003).
- Konnopka, A., König, H. Economic burden of anxiety disorders: a systematic review and meta-analysis. Pharmacoeconomics. 38 (1), 25-37 (2020).
- Batterham, P. J., et al. Effects of the COVID-19 pandemic on suicidal ideation in a representative Australian population sample-Longitudinal cohort study. Journal of Affective Disorders. 300, 385-391 (2022).
- Ismail, I. I., Kamel, W. A., Al-Hashel, J. Y. Association of COVID-19 pandemic and rate of cognitive decline in patients with dementia and mild cognitive impairment: a cross-sectional study. Gerontology and Geriatric Medicine. 7, 23337214211005223 (2021).
- . NIMH. Anxiety Disorders Available from: https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml (2018)
- Roy-Byrne, P. Treatment-refractory anxiety; definition, risk factors, and treatment challenges. Dialogues in Clinical Neuroscience. 17 (2), 191-206 (2015).
- Cassano, G. B., Baldini Rossi, N., Pini, S. Psychopharmacology of anxiety disorders. Dialogues in Clinical Neuroscience. 4 (3), 271-285 (2002).
- Garakani, A., et al. Pharmacotherapy of anxiety disorders: current and emerging treatment options. Frontiers in Psychiatry. 11, 595584 (2020).
- Hutson, P. H., Clark, J. A., Cross, A. J. CNS target identification and validation: avoiding the valley of death or naive optimism. Annual Review of Pharmacology and Toxicology. 57 (1), 171-187 (2017).
- Hart, P. C., Proetzel, G., Wiles, M. V., et al. Experimental models of anxiety for drug discovery and brain research. Mouse Models for Drug Discovery: Methods and Protocols. , 271-291 (2016).
- Scherma, M., Giunti, E., Fratta, W., Fadda, P. Gene knockout animal models of depression, anxiety and obsessive compulsive disorders. Psychiatric Genetics. 29 (5), 191-199 (2019).
- Liu, W. -. Z., et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nature Communications. 11 (1), 2221 (2020).
- Zheng, Z. -. H., et al. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain, Behavior, and Immunity. 91, 505-518 (2021).
- Toth, I., Neumann, I. D. Animal models of social avoidance and social fear. Cell and Tissue Research. 354 (1), 107-118 (2013).
- Wang, F., Kessels, H. W., Hu, H. The mouse that roared: neural mechanisms of social hierarchy. Trends in Neurosciences. 37 (11), 674-682 (2014).
- Endo, N., et al. Multiple animal positioning system shows that socially-reared mice influence the social proximity of isolation-reared cagemates. Communications Biology. 1 (1), 225 (2018).
- Netser, S., et al. Distinct dynamics of social motivation drive differential social behavior in laboratory rat and mouse strains. Nature Communications. 11 (1), 5908 (2020).
- Krimberg, J. S., Lumertz, F. S., Orso, R., Viola, T. W., de Almeida, R. M. M. Impact of social isolation on the oxytocinergic system: A systematic review and meta-analysis of rodent data. Neuroscience & Biobehavioral Reviews. 134, 104549 (2022).
- Mumtaz, F., Khan, M. I., Zubair, M., Dehpour, A. R. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomedicine & Pharmacotherapy. 105, 1205-1222 (2018).
- Ranade, S. Comforting in mice. Nature Neuroscience. 24 (12), 1640 (2021).
- Al Omran, A. J., et al. Social isolation induces neuroinflammation and microglia overactivation, while dihydromyricetin prevents and improves them. Journal of Neuroinflammation. 19 (1), 2 (2022).
- Watanabe, S., et al. Dihydromyricetin improves social isolation-induced cognitive impairments and astrocytic changes in mice. Scientific Reports. 12 (1), 5899 (2022).
- Silva, J., et al. Modulation of hippocampal GABAergic neurotransmission and gephyrin levels by dihydromyricetin improves anxiety. Frontiers in Pharmacology. 11, 1008 (2020).
- Porter, V. R., et al. Frequency and characteristics of anxiety among patients with Alzheimer’s disease and related dementias. Journal of Neuropsychiatry and Clinical Neuroscience. 15 (2), 180-186 (2003).
- Hossain, M. M., et al. Prevalence of anxiety and depression in South Asia during COVID-19: A systematic review and meta-analysis. Heliyon. 7 (4), 06677 (2021).
- . NHGRI. Knockout Mice Fact Sheet Available from: https://www.genome.gov/about-genomics/fact-sheets/Knockout-Mice-Fact-Sheet (2020)
- Takahashi, A. Social stress and aggression in murine models. Current Topics in Behavioral Neuroscience. 54, 181-208 (2022).
- Lam, R. W. Challenges in the treatment of anxiety disorders: beyond guidelines. International Journal of Psychiatry in Clinical Practice. 10, 18-24 (2006).
- Sullens, D. G., et al. Social isolation induces hyperactivity and exploration in aged female mice. PLoS One. 16 (2), 0245355 (2021).

