Separation of Follicular Cells and Oocytes in Ovarian Follicles of Zebrafish
Özet
Here, we present a simple method for separating follicular cells and oocytes in zebrafish ovarian follicles, which will facilitate investigations of ovarian development in zebrafish.
Abstract
Zebrafish has become an ideal model to study the ovarian development of vertebrates. The follicle is the basic unit of the ovary, which consists of oocytes and surrounding follicular cells. It is vital to separate both follicular cells and oocytes for various research purposes such as for primary culture of follicular cells, analysis of gene expression, oocyte maturation and in vitro fertilization, etc. The conventional method uses forceps to separate both compartments, which is laborious, time consuming and has high damage to the oocyte. Here, we have established a simple method to separate both compartments using a pulled glass capillary. Under a stereomicroscope, oocytes and follicular cells can be easily separated by pipetting in a pulled fine glass capillary (the diameter depends on the follicle diameter). Compared with the conventional method, this new method has high efficiency in separating both oocytes and follicular cells and has low damage to the oocytes. More importantly, this method can be applied to early-stage follicles including at the pre-vitellogenesis stage. Thus, this simple method can be used to separate follicular cells and oocytes of zebrafish.
Introduction
Zebrafish is a major model organism for the study of vertebrate development and physiology. The zebrafish can serve as a good model for studying the molecular mechanisms of ovarian development1,2,3. Many features of ovarian development are much conserved during evolution from fish to mammals1,2. Similar to the other vertebrates,zebrafish adults have asynchronous ovaries, containing ovarian follicles of all developmental stages4. The follicle is the fundamental reproductive element of the ovary. The follicle consists of the oocyte that is surrounded by one or several layers of somatic cells called follicular cells. The development of follicles depends on the bidirectional communication between oocytes and follicular cells5. It is vital to separate follicular cells and oocytes from ovarian follicles for different research purposes such as follicular cell primary culture, gene expression analysis, oocyte maturation, and in vitro fertilization.
Traditional separation methods include mechanical separation by forceps and enzymatic digestion6,7,8,9,10. However, the mechanical separation by forceps is time-consuming and laborious. It will also cause the high damage to the oocyte during separation. Although the enzyme digestion method is simple to operate and requires a short time, the treatment time and enzyme concentration should be validated, and the integrity and survival rate of the isolated oocytes are not ideal.Therefore, we have established a simple method to separate both compartments at different developmental stages using pulled glass capillary tubes.
Protocol
All of the procedures performed in fish experiments are in accordance with the regulations of the Animal Experimentation Ethics Committee of Northwest Normal University.
1. Preparations
- Animals
- Use adult female zebrafish with a body length of 4-6 cm.
NOTE: We used zebrafish from a local market. - Keep the zebrafish in a circulated water system with a 14 h light and 10 h dark cycle at about 28 °C.
- Feed fish twice daily with newly hatched brine shrimp.
- Use adult female zebrafish with a body length of 4-6 cm.
- Pulled glass capillary
- Use a 15 cm glass capillary and place its head in an alcohol burner to heat it. Hold one end of glass capillary by hand and hold the other end with forceps.
- After heating 5-10 seconds, the glass on the fire of alcohol burner become red and soft. Stretch the head of the glass capillary with forceps to make the capillary opening with required diameter.
NOTE: The diameter depends on the follicle diameters: previtellogenic (PV; about 0.30 mm in diameter), early vitellogenic (EV; about 0.40 mm in diameter), midvitellogenic (MV; about 0.50 mm in diameter), late vitellogenic (LV; about 0.60 mm in diameter) and full grown but immature (FG; about 0.65 mm in diameter). - Stretch the opening of glass capillary to the appropriate diameter, which is slightly smaller the size of separated follicles. The glass capillary with an opening much bigger than the size of ovarian follicles cannot perform separation, but the much smaller ones would break the follicles during separation. Practice the stretching of the glass capillary several times.
NOTE: Heating time depends on the size of glass capillary. Do not directly stretch the glass capillary on the fire of alcohol burner. It will break the glass capillary. - Cut the glass capillary with an ampoule cutter and break the glass capillary with forceps to obtain a smooth incision.
NOTE: A sharp or broken opening of the glass capillary would damage the follicles. - Using a 30 cm plastic tube, insert a 1 mL pipette tip with a filter element at one end, insert the pulled glass capillary at the end of the pipette, and connect a 200 µL pipette tip at another end.
2. Separation of zebrafish oocytes and follicular cells at different stages
- Dissection of the zebrafish ovary
- Fill an ice bucket to 4/5 full with slurry ice and add sufficient fish water to let slurry ice float. Wait 2-5 minutes, check the water temperature, and make sure the temperature is between 2-4 °C.
- Anesthetize adult females by placing fish in the ice water for at least 2-5 minutes, until fish stop gill movement. Decapitate the fish by severing the spinal cord using a sharp scissors to ensure death.
- Place the fish on the dissecting plate with forceps.
- Use dissecting scissors to cut as follows. Dissect from the cloaca to the gills along the midline of the abdomen. Cut from the back of the cloaca. Cut from the anterior tip to the dorsal side. Gently remove the skin and muscles on one side of the body. Expose the internal organs and take out the entire ovary with forceps.
- Immediately, place the ovaries gently into a 100 mm culture dish containing 60% Leibovitz's L-15 (L-15) medium.
- Isolation of ovarian follicles
NOTE: The staging system that we have adopted is based on the original definition of Selman et al.4.- Manually isolate follicles of different stages using a pair of fine forceps as described previously8.
- Group the ovarian follicles into the following stages: PV, EV, MV, LV and FG stages.
- Separation of follicular cells and oocyte from ovarian follicles
- Put the ovarian follicles at different stages into a clean Petri dish containing 60% L-15 medium.
- Using the pulled glass capillary from step 1.2, suck the follicles into the glass capillary 2-3 cm and blow them out.
NOTE: When the opening diameter of the glass capillaries is appropriate, the follicle can be separated from the oocyte by inhalation once.- If the follicular cell layer is attached to the oocyte firmly, repeat 2-3 times to accomplish a complete separation. Avoid multiple attempts to force a follicle through a smaller capillary, which would damage the follicle.
NOTE: In the process of inhalation and blowing out, the follicular layer falls off and separates from the oocyte, and finally, follicular cells and denuded oocytes are separated (Figure 1).
- If the follicular cell layer is attached to the oocyte firmly, repeat 2-3 times to accomplish a complete separation. Avoid multiple attempts to force a follicle through a smaller capillary, which would damage the follicle.
- Pool the denuded but intact oocytes and collect the surviving denuded oocytes for further assays.
- To investigate whether the follicular cells were separated from intact follicles, stain the intact follicles and separated oocytes with 4',6-diamidino-2-phenylindole (DAPI).
- Fix the samples in 4% buffered paraformaldehyde at room temperature (RT) for 1 h. Wash several times by PBS.
- Stain by DAPI at RT for 30 min. Washed several times with PBS. View and photograph with a fluorescence microscope (e.g., Leica DFC7000 T).
- To confirm the completed separation, fix the intact follicles and separated oocytes in 4% buffered paraformaldehyde overnight at 4 °C, and embed intissue-freezing medium (e.g., Leica) at -25 °C.
- Cut the fixed tissues on a microtome, and mount onto glass slides.
- Wash the sections in PBS and visualize the cell nuclei with DAPI. View and photograph on a fluorescence microscope.
3. In vitro maturation (IVM) and in vitro fertilization (IVF)
NOTE: The procedure of IVM of IVF was followed as described previously with minor modification 11,12,13.
- Prepare fresh maturation medium (+DHP medium) before ovary dissection from adult zebrafish female. To prepare the fresh maturation medium, add 9 mL of Leibovitz's L-15 medium, pH 9.0, to 15 mL conical tubes. Add 10 µL of 17α-20β-dihydroxy-4 pregnen-3-one (DHP) (5 mg/mL), 490 µL of dH2O, and 500 µL of 10% bovine serum albumin (BSA).
- Prepare Hank's solution and E3 solution in advance. Prepare Hanks' solution as described by Baars et al.14. Prepare E3 medium: 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, and 1-5% Methylene Blue.
- Perform dissection of zebrafish ovary and isolation of ovarian follicles as in steps step 2.
- Collect full grown stage follicles and use as immature oocytes. For each adult female zebrafish, at least 150-200 full grown stage follicles can be isolated. Select the intact and healthy follicles for IVM.
- Incubate the full-grown stage follicles in +DHP medium in 12-well plates, checking periodically to ensure that the oocytes remain intact. Remove any lysing oocytes with a Pasteur pipette.
NOTE: During the oocyte maturation, the oocytes will become progressively translucent. A majority of the oocytes become translucent after treatment of DHP for 2 h. This can be observed under a dissecting microscope with transmitted light optics. - Remove the outermost follicular cells from each matured oocyte as described in step 2.3.
- Transfer the denuded oocytes to a Petri dish with a few drops of culture medium and proceed to fertilization.
- Prepare the fresh sperm solution using testes dissected from at least three males in 500 µL of Hanks' solution. Put the sperm solution on ice, where it can keep its potency of fertilization for up to 2-3 h.
- Add 100 µL of sperm solution to denuded & matured oocytes on a Petri dish. Gently add the sperm solution among the eggs, and swirl the sperm and eggs together using a pipette tip.
- Immediately add 1 mL of E3 medium solution to activate the eggs and again gently swirl eggs and sperm using a pipette tip.
- After around 1 minute post-fertilization (mpf), flood the plate with E3 medium solution. Full chorion expansion can be observed within 10-15 mpf.
- Between 35-45 mpf, select the embryos that are undergoing symmetrical cleavage into the 2-cell stage, and which are therefore fertilized. Remove embryos that are not undergoing cell cleavage.
- Allow embryos to develop in the Petri dish at 28 °C, with a limit of 80 embryos per 10 cm plate. View and photograph the embryo development.
Representative Results
This method can be used to separate follicular cells and oocytes at different stages of ovarian follicle development in zebrafish. Figure 1 shows the separation of zebrafish oocytes and follicular cells from ovarian follicles using a capillary glass tube (Figure 1). To investigate whether the follicular cells were separated from intact follicles, the intact follicles and separated oocytes from different stages of follicles from the PV stage to the FG stage were stained with DAPI (50 µg/mL) at 37 °C for 30 minutes. The nucleus of follicular cells can be stained by DAPI. The blue signal of DAPI was observed in intact follicles but not denuded oocytes (Figure 2).This indicates that the oocytes and follicular cells of the follicle can be separated cleanly by this method. To further confirm the clear separation, the intact follicles and separated oocytes were cut into the histological sections. DAPI staining results showed that follicular cells were clearly removed in denuded oocytes (Figure 3). All these results suggest that this method can be used to separate follicular cells and oocytes in different stage follicles of zebrafish.
This method can be used for studying the oocyte maturation of zebrafish. To study the bidirectional communication between oocytes and follicular cells during the oocyte maturation, the denuded oocytes must be obtained for various assays. Using a pulled glass capillary, the denuded oocytes separated from full grown stage zebrafish follicles were found to undergo the spontaneous oocyte maturation without any treatment after 4 hours incubation, usually at least 30% separated oocytes survived and the rate of maturity of these survived oocytes could be up to 80% (Figure 4). These matured oocytes could undergo the full chorion expansion after placement into the water, suggesting that the follicular cells were completely removed (Figure 4). Thus, this method can be used to obtain the denuded oocytes for studying the oocyte maturation of zebrafish.
This method can be used for IVF in zebrafish. In vitro maturation (IVM) methods have been well established in zebrafish. Using these methods, the fully grown stage follicles can be induced into matured stage11,12,13,15,16. To further perform in vitro fertilization (IVF), the follicular layer of matured follicles still need to be manually removed. Here, IVM of zebrafish was induced by treatment of DHP. The follicular layer of matured follicles was removed by a capillary glass tube. These defolliculated oocytes could be fertilized and developed into the hatching stage (Figure 5). The result suggests that this method can be used for IVF in zebrafish. Usually around 10% of healthy embryos can be obtained from the matured oocytes.
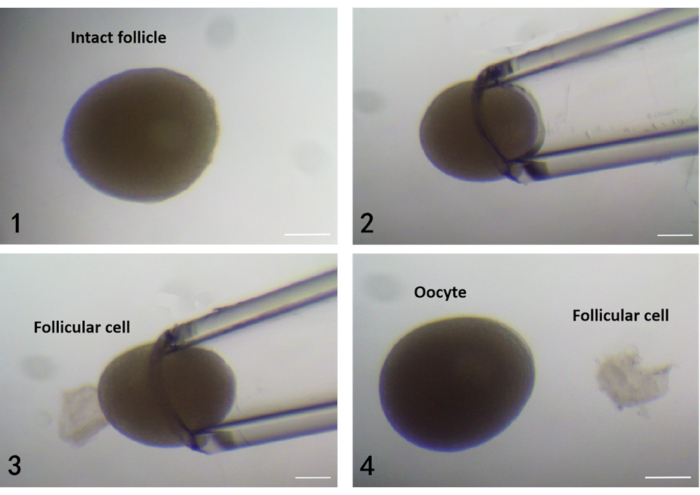
Figure 1. Separation of follicular cells and oocytes from zebrafish ovarian follicles using a pulled glass capillary. 1) The morphology of the intact follicle at FG stage. 2)Inhalation of the intact follicle into the pulled glass capillary. 3)The follicular cell is detached from the oocyte when the follicle is blown out from the pulled glass capillary. 4) Successful separation of follicular cells and oocyte. Scale bars: 200 µm. Please click here to view a larger version of this figure.
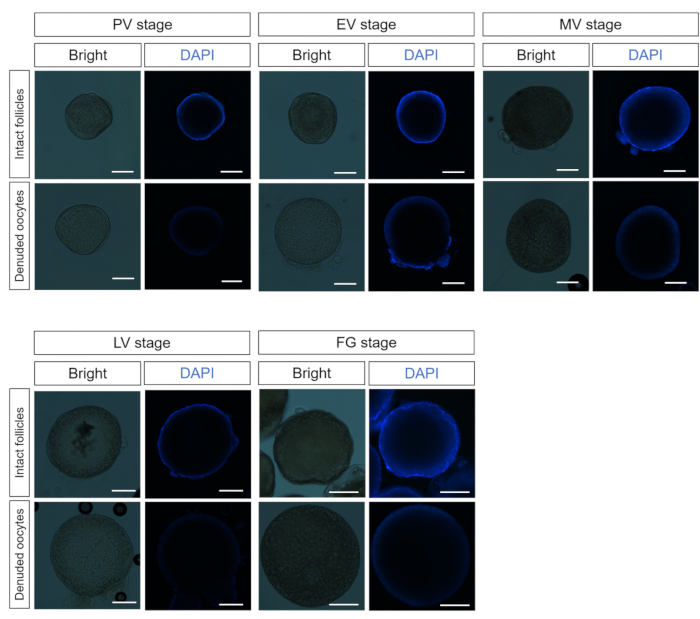
Figure 2. The morphology of intact follicles and denuded oocytes separated from different stages of follicles after DAPI staining. The intact follicles and separated oocytes from different stages of follicles from PV stage to FG stage were stained by DAPI. The blue signals of DAPI were observed in intact follicles but not denuded oocyte.Scale bars: 200 µm. Please click here to view a larger version of this figure.
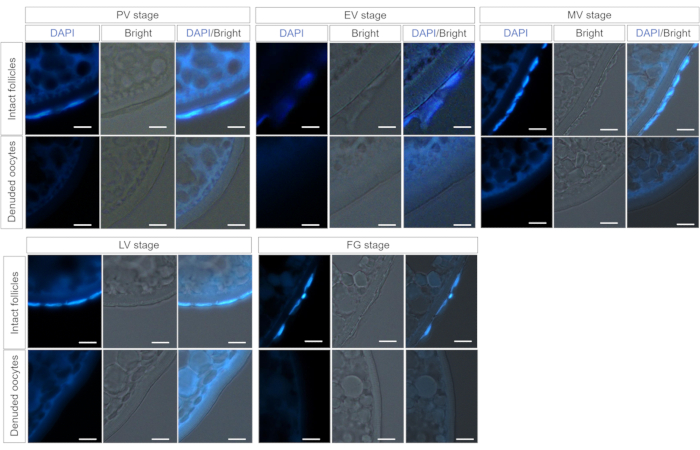
Figure 3. Histological sections of intact follicles and isolated oocytes after DAPI staining. Cell nuclei of follicular cells was visualized with DAPI staining. The blue signal can be observed. Follicular cells were clearly removed in denuded oocytes.Scale bars: 100 µm. Please click here to view a larger version of this figure.
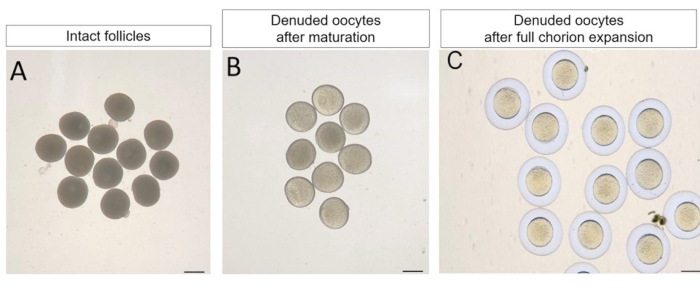
Figure 4. The maturation and full chorion expansion of denuded oocytes separated from full grown stage follicles using a pulled glass capillary. (A) The morphology of intact follicles at full grown stage follicles. (B) The denuded oocytes separated from full grown stage follicles can undergo spontaneous oocyte maturation without any treatment after 4 hours incubation. (C) The matured oocytes can undergo full chorion expansion after activation by water.Scale bar: 500 µm. Please click here to view a larger version of this figure.
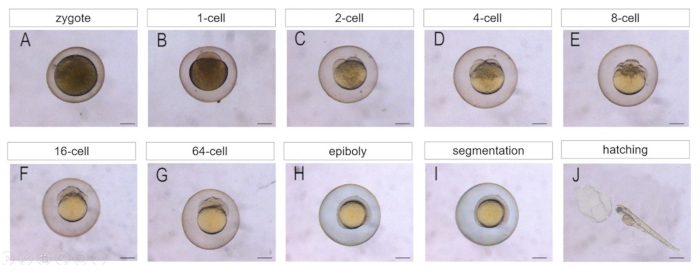
Figure 5. The morphology of early embryos obtained by IVM and IVF. IVM was induced by treatment of DHP (5 µg/mL) for 2 h. After IVM, the follicular cells were removed from the matured oocytes by a pulled glass capillary. The matured oocytes were fertilized by IVF. The development of embryos at different stages was viewed and photographed. Scale bars: (A-I) 200 µm, (J) 1 mm. Please click here to view a larger version of this figure.
Discussion
We describe here a novel method for the simple and rapid separation of follicular cells and oocytes from zebrafish ovarian follicles. This method has several advantages over the conventional method. Primary amongst these is the greatly increased ease of separation with high efficiency and effectiveness, as only a single and external manipulation is required. This point increases the applicability to researchers who are not good at microscopic anatomy. According to our experience, one can successfully separate follicular cells and oocytes from a fully grown stage follicle within 5-10 s using the glass capillary. However, at least 30-60 s is needed to do this separation using forceps. More importantly, usually at least 20% of the denuded oocytes separated by the glass capillary can undergo spontaneous oocyte maturation. In contrast, not beyond 1% of the denuded oocytes can undergo spontaneous oocyte maturation using forceps. Thus, the efficiency and effectiveness of the new method using s glass capillary is better than the previously established method using forceps. Second, this method can be used to separate follicular celld and oocyted of ovarian follicles at early developmental stages such as the PV, EV, and MV stages. Such separation cannot be performed at early-stage follicles using current methodologies.
There are also a few of limitations using this method. For example, the appropriate diameter of glass capillary opening is crucial for separation of follicular cells and oocytes in this method. An opening much bigger or smaller than the size of ovarian follicles would lead to the failure of separation. Thus, the opening diameter of glass capillary should be adjusted depending on the size of ovarian follicles, which needs some practice.
Separation of follicular cells and oocytes have several applications in studying ovarian development. Follicular cells and oocytes at different stages of ovarian follicles obtained by this method can be used to analyze gene expression. Follicular cells separated can be used for primary cell culture. Denuded oocytes separated are useful for investigating the crosstalk between follicular cells and oocytes, and supply a good model for studying the oocyte maturation. In addition, this method can be used to remove the follicular cells in some assays such as in vitro oocyte maturation or in vitro fertilization. The availability of a method that greatly increases the ease and speed of separation should facilitate wider exploitation of zebrafish ovarian follicles in the long-term goal of understanding the intricacies of ovarian development. In addition, the structure of ovarian follicles is similar among different fish species; thus, it is interesting to test whether this method can also be applied in other fishes.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This research work was supported by the National Natural Science Foundation of China [32060170, 31601205 and 31560334], visiting scholar project supported by China Scholarship Council and the fund of State Key Laboratory of Freshwater Ecology and Biotechnology [2020FB05].
Materials
| 17α,20β-DHP | Cayman | 16146-5 (5 mg) | |
| 24-well plate | Corning | 3524 | |
| Ampoule cutter | AS ONE | 5-124-22 1 bag (100 pieces) | |
| Anhydrous Na2HPO4 | Kaixin Chemical | 500 g | |
| Brine shrimp | Hongjie | 250 g | |
| CaCl2 | Beichen Fangzheng | 500 g | |
| Culture dish | Biosharp | BS-90-D (10PCS/PK) | |
| DAPI | Solarbio | S2110 (25mL) | |
| Dissecting Microscope | ZEISS | Stemi 305 | |
| Dissection forcep | VETUS | HRC30 | |
| Dissection scissor | Kefu | 160 mm | |
| Fluorescence Stereomicroscope | Leica | M205C | |
| Glass capillary | IWAKI | IK-PAS-5P (200 pcs/PACK) | |
| Hoechst 33342 | Solarbio | C0031 (1 mg) | |
| KCl | Beichen Fangzheng | 500 g | |
| KH2PO4 | Kaixin Chemical | 500 g | |
| Leibovitz’s L-15 medium | Gibco | 41300-039 (10×1L) | |
| MgSO4•7H2O | Beichen Fangzheng | 500 g | |
| Micropipette tips | Axygen | MCT-150-C | |
| NaCl | Beichen Fangzheng | 500 g | |
| NaHCO3 | Beichen Fangzheng | 500 g | |
| Penicilia-streptomycia | Gibco | #15140122 (100 mL) | |
| Stereomicroscope | ZEISS | Discover.v20 |
Referanslar
- Clelland, E., Peng, C. Endocrine/paracrine control of zebrafish development. Molecular and Cellular Endocrinology. 312, 42-52 (2009).
- Ge, W. Intrafollicular paracrine communication in the zebrafish ovary: the state of the art of an emerging model for the study of vertebrate folliculogenesis. Molecular and Cellular Endocrinology. 237, 1-10 (2005).
- Li, J., Ge, W. Zebrafish as a model for studying ovarian development: Recent advances from targeted gene knockout studies. Molecular and Cellular Endocrinology. 507, 1-19 (2020).
- Selman, K., Wallace, R. A., Sarka, A., Qi, X. Stages of oocyte development in the zebrafish, Brachydanio rerio. Journal of Morphology. 218, 203-224 (1993).
- Matzuk, M. M., Burns, K. H., Viveiros, M. M., Eppig, J. J. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 296, 2178-2180 (2002).
- Liu, L., Ge, W. Growth differentiation factor 9 and its spatiotemporal expression and regulation in the zebrafish ovary. Biology of Reproduction. 76, 294-302 (2007).
- Zhou, R., Tsang, A. H., Lau, S. W., Ge, W. Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors in the zebrafish ovary: evidence for potentially dual roles of PACAP in controlling final oocyte maturation. Biology of Reproduction. 85, 615-625 (2011).
- Li, J., Liu, Z., Wang, D., Cheng, C. H. K. Insulin-like growth factor 3 is involved in oocyte maturation in zebrafish. Biology of Reproduction. 84, 476-486 (2011).
- Pang, Y., Thomas, P. Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Gelişim Biyolojisi. 342, 194-206 (2010).
- Peyton, C., Thomas, P. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio). Biology of Reproduction. 85, 42-50 (2011).
- Welch, E. L., Eno, C. C., Nair, S., Lindeman, R. E., F, P. Functional manipulation of maternal gene products using in vitro oocyte maturation in zebrafish. Journal of Visualized Experiments. (122), e55213 (2017).
- Nair, S., Lindeman, R. E., Pelegri, F. In vitro oocyte culture-based manipulation of zebrafish maternal genes. Developmental Dynamics. 242, 44-52 (2013).
- Seki, S., et al. Development of a reliable in vitro maturation system for zebrafish oocytes. Reproduction. 135, 285-292 (2008).
- Baars, D. L., Takle, K. A., Heier, J., Pelegri, F. Ploidy manipulation of zebrafish embryos with heat shock 2 treatment. Journal of Visualized Experiments. , e54492 (2016).
- Xie, S. L., et al. A novel technique based on in vitro oocyte injection to improve CRISPR/Cas9 gene editing in zebrafish. Scientific Reports. 6, 34555 (2016).
- Li, J., Bai, L., Liu, Z., Wang, W. Dual roles of PDE9a in meiotic maturation of zebrafish oocytes. Biochemical and Biophysical Research Communications. , (2020).

