Isolation and Differentiation of Primary Myoblasts from Mouse Skeletal Muscle Explants
Özet
Myoblasts are proliferating precursor cells that differentiate to form polynucleated myotubes and eventually skeletal muscle myofibers. Here, we present a protocol for efficient isolation and culture of primary myoblasts from young adult mouse skeletal muscles. The method enables molecular, genetic, and metabolic studies of muscle cells in culture.
Abstract
Primary myoblasts are undifferentiated proliferating precursors of skeletal muscle. They can be cultured and studied as muscle precursors or induced to differentiate into later stages of muscle development. The protocol provided here describes a robust method for the isolation and culture of a highly proliferative population of myoblast cells from young adult mouse skeletal muscle explants. These cells are useful for the study of the metabolic properties of skeletal muscle of different mouse models, as well as in other downstream applications such as transfection with exogenous DNA or transduction with viral expression vectors. The level of differentiation and metabolic profile of these cells depends on the length of exposure, and composition of the media used to induce myoblast differentiation. These methods provide a robust system for the study of mouse muscle cell metabolism ex vivo. Importantly, unlike in vivo models, the methods described here provide a cell population that can be expanded and studied with high levels of reproducibility.
Introduction
While often cited as an indication of overall metabolic health, multiple studies have shown that body mass index (BMI) in older adults is not consistently associated with higher risk of mortality. To date, the only factor shown to be consistent with reduced mortality in this population is increased muscle mass1. Muscle tissue represents one of the largest sources of insulin-sensitive cells in the body, and is therefore critical in the maintenance of overall metabolic homeostasis2. Activation of skeletal muscle tissue via exercise is associated with increases in both local insulin sensitivity and overall metabolic health3. While in vivo models are essential for studying muscle physiology and the impact of muscle function on integrated metabolism, primary cultures of myotubes provide a tractable system that reduces the complexity of animal studies.
Myoblasts derived from post-natal muscles can be used to study the impact of numerous treatment and growth conditions in a highly reproducible manner. This has long been recognized and several methods for myoblast isolation and culture have been described4,5,6,7,8,9. Some of these methods use neonatal muscles and yield relatively low numbers of myoblasts5,8, requiring several animals for larger scale studies. Also, most widely used methods for culturing myoblasts use "pre-plating" to enrich for myoblasts, which are less adherent than other cell types. We have found the alternative enrichment method described here to be much more efficient and reproducible for enriching a highly proliferative myoblast population. In summary, this protocol enables the isolation of highly proliferative myoblasts from young adult muscle explants, via outgrowth into culture media. Myoblasts can be harvested repeatedly, over several days, rapidly expanded, and induced to differentiate into myotubes. This protocol reproducibly generates a large number of healthy myoblast cells that robustly differentiate into spontaneously twitching myotubes. It has enabled us to study metabolism and circadian rhythms in primary myotubes of mice of a variety of genotypes. Finally, we include methods for preparing myotubes for the study of oxidative metabolism, using measurements of oxygen consumption rates in 96-well plates.
Protocol
This protocol follows the animal care guidelines of Scripps Research.
1. Collection and Processing of Muscle Tissue Explants
- The day prior to dissection, sterilize all dissection equipment (forceps, razor blades, and scissors) and prepare all required media: phosphate buffered saline (PBS), MB Plating media (12.5 mL of DMEM, 12.5 mL of HAMS F12, 20 mL of heat-inactivated fetal bovine serum (FBS), 5 mL of amniotic fluid medium supplement), and Coating Solution (24 mL of DMEM, 24 mL of HAMS F12, 1.7 mL of collagen, 1 mL of matrigel).
- The day of dissection, coat one 6-cm dish with Coating Solution for each muscle to be dissected. Add 2 mL of Coating Solution to the surface of each plate, shake gently to create an even coat on the surface, and incubate the plates with solution at 4 °C for 1 h.
- Remove Coating Solution from the plates and return to the stock solution.
NOTE: Coating Solution can be reused for up to six months and should be stored at 4 °C. - Rinse the plates twice with 2 mL of PBS to remove unbound collagen and matrigel.
- Place the plates in a 37 °C tissue culture incubator during dissections.
- Prepare a moist chamber by placing 2-3 sheets of thick absorbent paper into a plastic bag or a sterile 15 cm dish and use a pipette to wet the surface of the paper with sterile water.
- Place the chamber under UV light for 5 min to sterilize.
- Dissect desired muscles from a 4 to 8-week old mouse. To sterilize muscle tissue, rinse gently in PBS containing 40 μg/mL gentamicin.
NOTE: For quadriceps and gastrocnemius muscles, plate one muscle per plate. For soleus, plantaris and EDL, combine muscles from both legs in one plate. - Use sterile forceps to transfer the muscle to a sterile 10 cm non-coated Petri dish. Add 0.5-1.0 mL of Plating media over the muscle such that the tissue is moist but not floating (Figure 1A).
- Use a sterile scalpel or razor blade to gently slice the muscle into small fragments (approximately 1-3 mm3).
NOTE: It is important to minimize handling the muscle tissue for best results. - Use forceps or a pipette to transfer the muscle fragments onto the surface of a pre-coated 6-cm plate. Very gently overlay an additional 0.8 mL of Plating Media over the tissue. There should be enough media to keep the tissue pieces hydrated but not floating (Figure 1B).
- Place the 6-cm dishes containing the muscle fragments inside the moist chamber and return to an incubator (37 °C, 5% CO2) for 48 h (Figure 1C).
NOTE: It is vital that muscle fragments adhere to the surface of the plate to allow for myoblast outgrowth. Do not move the plates/chamber for at least 48 h. - After 48 h, carefully check the plates to ensure that muscle fragments have adhered. Overlay with 2 mL of Plating Media, taking care not to dislodge the fragments.
NOTE: If there is visible contamination or debris on the plates, carefully wash the muscle pieces. Plate 2 mL of PBS/gentamicin solution at the edge of the plate and tip gently to wash over the muscle tissue. Remove the PBS and repeat the wash step. Following the second wash, overlay 2 mL of Plating Media. - Keep the plates in the 37 °C incubator for up to an additional 3 days (5 days from original dissection) before harvesting myoblasts. Check every other day for outgrowth of myoblasts.
NOTE: The appearance of cells emerging from muscle explants will be variable and heterogeneous. Primary myoblasts appear as small, round, and bright cells. However, it is neither necessary nor reliable to identify them by their appearance at this stage (Figure 2).
2. Harvesting Outgrowing Myoblasts
- Prepare and pre-warm Myoblast Media (17.5 mL of DMEM, 17.5 mL of HAMS F12, 10 mL of FBS, 5 mL of amniotic fluid medium supplement), trypsin, and PBS/gentamicin. Coat one T25 flask per muscle group being harvested with Coating Solution and as described in step 1.2 (see Table 1).
- Remove Plating Media and gently rinse the muscle explants with 2 mL of PBS/gentamicin. Quickly remove PBS (rinse one plate at a time and do not let the plate sit in PBS at this step).
- Gently add 1 mL of PBS/gentamicin and place the plate in the 37 °C incubator for 1 min.
- Use a P1000 pipette to collect PBS/cells in a 15 mL centrifuge tube.
- Add 1 mL of trypsin to the plate and return to the 37 °C incubator for 3 min. Gently tap the plates to dislodge myoblasts. Collect the trypsin/cells and combine with the PBS collection. Add 8 mL of Myoblast Media to the centrifuge tube and gently invert to mix.
- Gently overlay 2 mL of Plating Solution on the muscle plates and return to the 37 °C incubator.
- Spin the centrifuge tubes containing cells in a centrifuge for 3 min at 200 x g.
- Aspirate the supernatant to ~1 mL, being careful to avoid cell pellet. Gently add Myoblast Media (see Table 1) and transfer the cells to the pre-coated flask and place in the 37 °C incubator. This is the P0 harvest. If observed under a microscope, there may be very few cells (Figure 3).
- Repeat the harvest described above every other day up to three times. After the third harvest, discard the explants.
3. Expansion and Enrichment of Proliferating Myoblasts
NOTE: The P0 harvest will be heterogeneous (~60% myoblasts). The next 2 passages use PBS to selectively harvest myoblasts. Many of the more adherent cells will be left behind and the rapidly proliferating myoblasts will be ≥95% pure within 2 passages. Once myoblasts are established, they should be maintained at a low density to avoid spontaneous differentiation.
- For each T25 flask of ~40-50% confluent cells from section 2, coat one T75 flask with 5 mL of Coating Solution and place at 4 °C for 1-4 h.
- Remove Coating Solution from the flasks and return to stock solution. Rinse the flasks twice with 2 mL of PBS/gentamicin and place in the 37 °C incubator.
- Aspirate the media from P0 myoblast T25 flasks. Rinse the cells briefly with 2 mL of warm PBS/gentamicin. Aspirate PBS from the flask.
NOTE: The purpose of this step (3.3) is to reduce the possibility of bacterial contamination. If performed quickly and gently, it should not result in loss of myoblasts. It can be omitted to maximize myoblast preservation if desired. - Pipette 2 mL of warm PBS (not trypsin) into each flask containing myoblasts. Place the flasks with PBS into the 37 °C incubator for 3 min.
NOTE: Myoblasts should easily detach from flasks with PBS. Using PBS rather than trypsin at this step is critical for reducing contamination of the myoblast population with other cell types. - Remove the cells from the 37 °C incubator and firmly tap side of the flasks to dislodge the cells. Check under a light microscope for freely floating myoblasts.
- Place the flasks upright in a tissue culture hood and rinse the bottom of the flasks with 10 mL of Myoblast Media 2-3 times to ensure all cells are dislodged.
- Collect the cell/media mixture in a 15 mL centrifuge tube. Centrifuge for 3 min at 200 x g.
- Aspirate the media to around 1 mL, being careful to avoid the cell pellet. Gently add an appropriate volume of Myoblast Media to centrifuge tube and gently mix.
- Distribute the cell mixture to new T75 flasks. Add 10 mL of Myoblast Media to each new T75 flask. Gently shake the flasks horizontally to distribute the cells and place in the 37 °C incubator overnight.
- Two days later, passage once more with PBS, splitting each T75 flask into three T75 flasks.
NOTE: Do not allow myoblasts to become more than 50%-60% confluent, as this would cause them to start differentiating and lose prolifaterive capacity. - For additional passages, use trypsin. Passaging twice with PBS yields >95% myoblasts; attempts to further improve the purity tends to result in poorer differentiation.
4. Differentiation of Primary Myoblasts to Myotubes
- Plate P2 (or later passage) myoblasts in Myoblast Media on coated plates (see Table 1 for suggested coating and plating volumes and cell numbers). Two or three days later, when cells are at 70-80% confluency, change the media to Differentiation Media (24 mL of DMEM, 24 mL of HAMS F12, 1.5 mL of heat inactivated horse serum, 0.5 mL of Insulin-Selenium-Transferrin).
- Change Differentiation Media every other day during differentiation of primary myoblasts into myotubes. Differentiation is typically complete by Day 4-5 and will be marked by elongated, fused cells that spontaneously twitch. Perform experiments on differentiated myotubes within 6 days. Typically cells are assayed five or six days after initiating differentiation.
5. Measuring Oxygen Consumption Rate in Myoblasts or Myotubes in 96-well Plates
- Coat 96-well plates with 25 μL of coating solution per well. Centrifuge the plates at 58 x g for 1 min to remove any bubbles.
- Incubate the plates in coating solution for 1-4 h at 4 °C. Use multi-channel pipette to remove the coating solution and wash three times in 25 μL of cold PBS/gentamicin. Centrifuge at least one of these washes to ensure that no bubbles are trapped.
- Add 40 μL of Differentiation Media to each well. Centrifuge the media on the coated plate with no cells for 1 min at 58 x g to avoid bubbles and remove surface tension to get a uniform layer of media.
- Add cells suspended in an additional 40 μL of media to each well. Spin again at 58 x g.
NOTE: Consistency in plating is important for best results and the number of cells plated per well should be optimized for each experimental setup, and will likely be in the range of ~10,000-25,000 myoblasts plated in differentiation media per well of a 96-well plate. - Gently change the media daily during differentiation. Do not aspirate the media but rather remove it with a pipette, leaving a small volume behind to avoid dislodging the cells or exposing them to the air. For example, replace 50 μL at a time for three repeats rather than all 80 μL at once.
- Use 8-15 wells per condition. It may be necessary to omit some wells if cells do not form a uniform layer of myotubes.
NOTE: Omit wells on the edges of the plate because they are highly susceptible to evaporation. Vary the plate setup for experimental replicates to avoid systematic errors. - Perform desired assay on differentiated myotubes within 1-2 days of full differentiation (Day 4-6 from start of differentiation). Recommended instruments and reagents for measurement of oxygen consumption rates are listed in the Table of Materials.
Representative Results
Following Section 1 of the provided protocol should yield primary cells emerging from the explants that will be visible under a standard light microscope (Figure 2). A heterogeneous cell population will be seen growing out of and surrounding each muscle tissue explant. Myoblasts will appear as small, round, bright spheres. Following Section 2 of the protocol will yield early harvests of myoblasts from tissue explants, which will contain few cells and will be heterogeneous (Figure 3). Section 3 of the protocol describes passaging early harvests with PBS (rather than trypsin), which will provide a relatively pure population of myoblasts for further culturing. Following Section 4 of the protocol will yield fully differentiated myotubes for further experimental manipulation. Differentiation of myoblasts typically takes 4-6 days, during which the morphology of the cells will change from single, round spheres to elongated, fused, long multinucleated fibers (Figure 4). Following section 5 of the protocol will produce differentiated myotubes in 96-well plates to enable a variety of metabolic characterizations based on the changes in oxygen consumption and extracellular acidification rates10 (Figure 5).
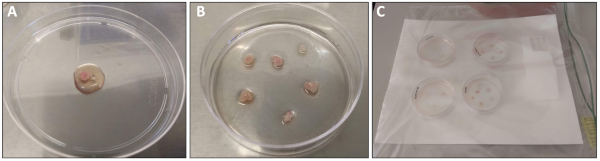
Figure 1: Dissection and processing of quadriceps muscle. (A) Quadriceps muscle that has been freshly dissected and rinsed with PBS prior to transfer to a 10 cm dish. 1 mL of plating media has been overlaid for processing. (B) Quadriceps muscle tissue pieces after transfer to pre-coated 6 cm plate. (C) 6 cm plates inside moist chamber prior to placement in the 37 °C incubator. Please click here to view a larger version of this figure.
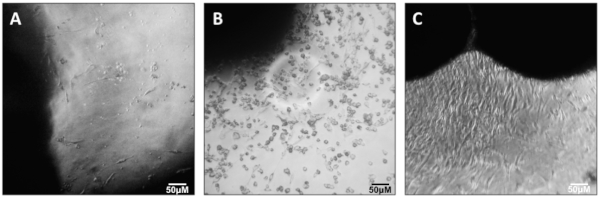
Figure 2: Outgrowth of myoblasts. Outgrowth of myoblasts from quadriceps muscle explants. Please click here to view a larger version of this figure.
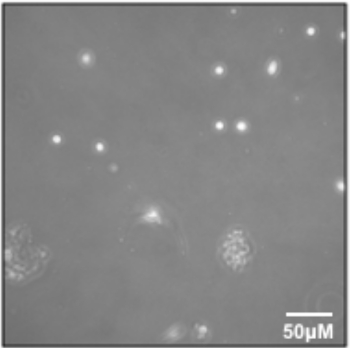
Figure 3: Early passage myoblasts. P0 myoblasts after transfer and attachment to T25 flask. Please click here to view a larger version of this figure.
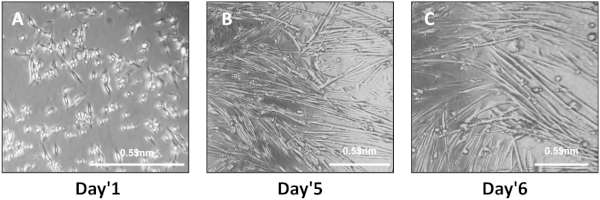
Figure 4: Plating and differentiation of primary myotubes. (A) Myoblasts one day after initiating exposure to Differentiation Media. (B,C) Differentiated myotubes five (B) or six (C) days after initiating differentiation. Please click here to view a larger version of this figure.
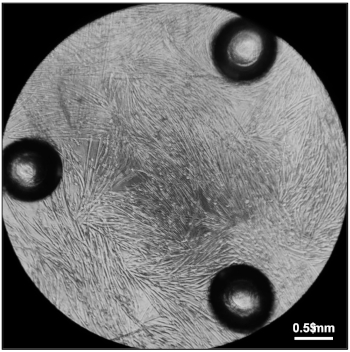
Figure 5: Myotubes ready for measurement of oxygen consumption rates. Fully differentiated myotubes five days after plating 20,000 myoblasts in Differentiation Media in each well of a 96-well cell culture microplate. Please click here to view a larger version of this figure.
Discussion
Skeletal muscle is vital for the establishment and maintenance of metabolic homeostasis11. The study of muscle physiology is complicated by interindividual variability, as well as difficulty in obtaining samples, particularly in the case of human studies. Cultured primary myotubes have been shown to recapitulate many features of muscle physiology, including calcium homeostasis12, regeneration of damaged muscle tissue5, metabolic alterations in response to exercise13, and alterations to metabolism resulting from diseases such as diabetes14. Primary culture of myoblasts and myotubes from mice enables investigation of muscle cells harboring well defined genetic manipulations, and provides a complement to studies of myotubes derived from human muscle biopsies12,15,16. Therefore, methods for isolation and culture of mouse primary myoblasts and myotubes are essential to enable reproducible, high-throughput investigation of muscle cell function ex vivo. The protocol described here allows for the establishment and study of primary mouse myoblasts and myotubes under a variety of experimental manipulations.
While previous protocols have described the isolation of muscle stem cells from explant cultures, this protocol provides a method for the successful isolation of myoblasts from multiple different types of muscle tissue. In addition, this method yields a significantly larger population of stem cells for further experimental manipulation. Further, this method has been validated as yielding differentiated myotubes that express markers of mature muscle cells17, and exhibit normal physiology, such as circadian rhythms17 and mitogen activated protein kinase (MAPK) signal transduction18.
The critical steps in the protocol are the dissection and processing of the muscle tissue explants, as well as the avoidance of contamination between harvests. Care should be taken to avoid over-processing of the tissues. While smaller pieces of muscle yield larger numbers of myoblasts, excessive cutting of the muscle could prevent stem cell outgrowth. While it is important not to dislodge the explants once they are plated, careful washing of the plates with PBS/gentamicin is critical for reducing contamination. Harvested myoblasts may be frozen as P2 cells in cryovials using a 10% DMSO/90% Myoblast Media mixture. While myoblasts do not need to be maintained at a high density to facilitate growth, it is advised that cells are frozen at 40-50% confluence. Typically, one T75 flask yields 4 cryovials of cells.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
The authors are grateful to Dr. Matthew Watt at the University of Melbourne and Dr. Anastasia Kralli at Johns Hopkins University for assistance adopting this protocol based on the work of Mokbel et al.6. We also thank Dr. Sabine Jordan for assistance developing and adopting this protocol in our laboratory. This work was funded by the National Institutes of Health R01s DK097164 and DK112927 to K.A.L.
Materials
| Coating Solution: | |||
| DMEM | Gibco | 10569010 | Always add gentamicin (1:1000 by volume) prior to use; 24 mL |
| HAMS F12 | Lonza | 12-615F | Always add gentamicin (1:1000 by volume) prior to use; 24 mL |
| Collagen | Life Technologies | A1064401 | 1.7 mL |
| Matrigel | Fisher | CB40234A | 1 mL |
| Plating Media: | |||
| DMEM | Gibco | 10569010 | Always add gentamicin (1:1000 by volume) prior to use; 12.5 mL |
| HAMS F12 | Lonza | 12-615F | Always add gentamicin (1:1000 by volume) prior to use; 12.5 mL |
| Heat Inactivated FBS | Life Technologies | 16000044 | 20 mL; can be purchased as regular FBS and heat-inactivated by placing in a 40 °C water bath for 20 minutes |
| Amniomax | Life Technologies | 12556023 | 5 mL |
| Myoblast Media: | |||
| DMEM | Gibco | 10569010 | Always add gentamicin (1:1000 by volume) prior to use; 17.5 mL |
| HAMS F12 | Lonza | 12-615F | Always add gentamicin (1:1000 by volume) prior to use; 17.5 mL |
| Heat Inactivated FBS | Life Technologies | 16000044 | 10 mL; can be purchased as regular FBS and heat-inactivated by placing in a 40 °C water bath for 20 minutes |
| Amniomax | Life Technologies | 12556023 | 5 mL |
| Differentiation Media: | |||
| DMEM | Gibco | 10569010 | Always add gentamicin (1:1000 by volume) prior to use; 24 mL |
| HAMS F12 | Lonza | 12-615F | Always add gentamicin (1:1000 by volume) prior to use; 24 mL |
| Heat Inactivated Horse Serum | Sigma | H1138 | 1.5 mL |
| Insulin-Selenium-Transferrin | Life Technologies | 41400045 | 0.5 mL |
| Other Materials: | |||
| PBS | Gibco | 14040133 | |
| Gentamicin | Sigma | G1397 | |
| TrypLE | Gibco | 12604013 | |
| DMSO | Sigma | 472301 | Prepare as 10% DMSO in Myoblast Media for freezing cells |
| Forceps | Any | ||
| Razor Blades | Any | ||
| Scissors | Any | ||
| Whatman paper | VWR | 21427-648 | |
| 60 mm plate | VWR | 734-2318 | |
| 10 cm plate | VWR | 25382-428 (CS) | |
| T25 Flasks | ThermoFisher | 156367 | |
| T75 Flasks | ThermoFisher | 156499 | |
| Centrifuge Tubes (15mL) | BioPioneer | CNT-15 | |
| Oxygen Consumption Rates: | |||
| Seahorse XFe96 Analyzer | Agilent | Seahorse XFe96 Analyzer | Instrument used to measure oxygen consumption rates read out by acidification of the extracellular media |
| Seahorse XFe96 FluxPak | Agilent | 102416-100 | 96-well plates for use in XFe96 Analyzer |
| Seahorse XF Cell Mito Stress Test Kit | Agilent | 103015-100 | components may be purchased from other suppliers once assay is established; some recommendations are listed below |
| Seahorse XF Palmitate-BSA FAO substrate | Agilent | 102720-100 | components may be purchased from other suppliers once assay is established; some recommendations are listed below |
| Palmitic acid | Sigma | P5585-10G | for measurement of fatty acid oxidation |
| carnitine | Sigma | C0283-5G | for measurement of fatty acid oxidation |
| Etomoxir | Sigma | E1905 | for measurement of fatty acid oxidation |
| BSA | Sigma | A7030 | used as control or in conjugation with palmitic acid for use in measurement of fatty acid oxidation |
Referanslar
- Srikanthan, P., Karlamangla, A. S. Muscle mass index as a predictor of longevity in older adults. American Journal of Medicine. 127 (6), 547-553 (2014).
- Lee-Young, R. S., Kang, L., Ayala, J. E., Wasserman, D. H., Fueger, P. T. The physiological regulation of glucose flux into muscle in vivo. Journal of Experimental Biology. 214 (2), 254-262 (2010).
- Sjøberg, K. A., et al. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes. 66 (6), 1501-1510 (2017).
- Girgis, C. M., Clifton-Bligh, R. J., Mokbel, N., Cheng, K., Gunton, J. E. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology. 155 (2), 347-357 (2014).
- Smith, J., Merrick, D. Embryonic skeletal muscle microexplant culture and isolation of skeletal muscle stem cells. Methods in Molecular Biology. 633, 29-56 (2010).
- Mokbel, N., et al. K7del is a common TPM2 gene mutation associated with nemaline myopathy and raised myofibre calcium sensitivity. Brain. 136 (2), 494-507 (2013).
- Yaffe, D., Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 270, 725-727 (1977).
- Rando, T. A., Blau, H. M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. Journal of Cell Biology. 125 (6), 1275-1287 (1994).
- Musarò, A., Carosio, S. Isolation and Culture of Satellite Cells from Mouse Skeletal Muscle. Methods in Molecular Biology. 1553, 155-167 (2017).
- Smolina, N., Bruton, J., Kostareva, A., Sejersen, T. Assaying mitochondrial respiration as an indicator of cellular metabolism and fitness. Methods in Molecular Biology. 1601, 79-87 (2017).
- Elliott, B., Renshaw, D., Getting, S., Mackenzie, R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiologica. 205 (3), 324-340 (2012).
- Smolina, N., Kostareva, A., Bruton, J., Karpushev, A., Sjoberg, G., Sejersen, T. Primary murine myotubes as a model for investigating muscular dystrophy. BioMed Research International. , (2015).
- Nedachi, T., Fujita, H., Kanzaki, M. Contractile C 2 C 12 myotube model for studying exercise-inducible responses in skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 295 (5), E1191-E1204 (2008).
- Chen, M. B., et al. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. Journal of Clinical Endocrinology and Metabolism. 90 (6), 3665-3672 (2005).
- Douillard-Guilloux, G., Mouly, V., Caillaud, C., Richard, E. Immortalization of murine muscle cells from lysosomal α-glucosidase deficient mice: A new tool to study pathophysiology and assess therapeutic strategies for Pompe disease. Biochemical and Biophysical Research Communications. 388 (2), 333-338 (2009).
- Varga, B., et al. Myotube elasticity of an amyotrophic lateral sclerosis mouse model. Scientific Reports. 8 (1), 5917 (2018).
- Kriebs, A., et al. Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proceedings of the National Academy of Sciences. 114 (33), 8776-8781 (2017).
- Cho, Y., et al. Perm1 enhances mitochondrial biogenesis, oxidative capacity, and fatigue resistance in adult skeletal muscle. FASEB Journal. 30 (2), 674-687 (2016).

