Assessing the Autonomic and Behavioral Effects of Passive Motion in Rats using Elevator Vertical Motion and Ferris-Wheel Rotation
Özet
Protocols are presented to assess the autonomic and behavioral effects of passive motion in rodents using elevator vertical motion and Ferris-wheel rotation.
Abstract
The overall goal of this study is to assess the autonomic and behavioral effects of passive motion in rodents using the elevator vertical motion and Ferris-wheel rotation devices. These assays can help confirm the integrity and normal functioning of the autonomic nervous system. They are coupled to quantitative measures based on defecation counting, open-field examination, and balance beam crossing. The advantages of these assays are their simplicity, reproducibility, and quantitative behavioral measures. The limitations of these assays are that the autonomic reactions could be epiphenomena of non-vestibular disorders and that a functioning vestibular system is required. Examination of disorders such as motion sickness will be greatly aided by the detailed procedures of these assays.
Introduction
Motion sickness (MS) due to abnormal visuo-vestibular stimulation leads to autonomic reaction, eliciting symptoms such epigastric discomfort, nausea and/or vomiting1. According to current theories, motion sickness may be caused by a sensory conflict or neuronal mismatch from receiving integrated motion information that differs from the anticipated internal model of the environment2,3 or postural instability as would occur on a yawing ship4,5. Despite significant advances in the field of motion sickness and vestibular autonomic functioning6,7,8,9,10,11,12, future research can be aided by standardized evaluation protocols. Assessing the autonomic effects of standard passive motions will greatly benefit investigations into the causes and prevention of motion sickness. The overall goal of this study is to assess the autonomic and behavioral effects of passive motion in rodents. Animal models, such as rodents, allow easy experimental manipulation (e.g., passive motion and pharmaceutical) and behavioral evaluation, which can be used to study the etiology of motion sickness. Here, we present a detailed battery for testing the effects of passive motion and the integrity of vestibular functioning.
The present study details two assays, elevator vertical motion (EVM) and Ferris-wheel rotation (FWR), that induce autonomic reactions to the passive motion. The assays are coupled to three quantitative behavioral measures, the balance beam (on mice13 and rats14,15,16,17), open-field examination, and defecation counting. The EVM (similar to the pitch and roll of a ship encountering a wave) assesses vestibular functioning by stimulating the otolith sensory organs that encode linear accelerations (i.e., the saccule that responds to movements in the vertical plane)18. The FWR (centrifugal rotation or sinusoidal motion) device stimulates the otolith organs by linear acceleration and the semicircular canals by angular acceleration19,20. The Ferris-wheel/centrifugal rotation device is unique in its autonomic assessment. To date, the only similar device in the literature is the off-vertical axis rotation (OVAR) turntable, which is used to examine the vestibulo-ocular reflex (VOR)18,21,22, conditioned avoidance23,24, and the effects of hypergravity25,26,27. The EVM assay and the FWR device assay induce vestibular stimulation leading to autonomic reactions. We couple the EVM and FWR to quantitative measurements such as balance beam, defecation counting, and open-field analysis28,29,30, to ensure robust and reproducible results. Similar to those previously described in mice13 and rats14,15,16,17, the balance beam assay is a 1.0 m long beam suspended 0.75 m from the ground between two wooden stools using a simple black-box modification at the goal end (finish). The balance beam has been used to assess anxiety (obscure black box)14,17, traumatic injury15,16,17, and here, autonomic reactions affecting balance. We have performed defecation counting for assessing the autonomic response in the motion sickness model previously, and it is a reliable quantitative measurement that is easily performed and unequivocally assessed6,8,9,11. The open-field analysis employs a simple black box open-field behavior assessment using Ethovision28, Bonsai30, or a simple video analysis in Matlab29 to quantify behavior such as motion. In the current protocol, we use the total distance traveled, but we note several different paradigms exist (e.g., elongation, zone of movement, velocity, etc.)28,29,30. Collectively, these procedures form a short battery of assessments for the examination and evaluation of autonomic reactions to passive motion, for example in motion sickness6,7,8,9,10,11. The present assays can be adapted to a variety of animal models.
Protocol
The present study and procedures were approved by the Ethics Committee for Animal Experimentation of the Second Military Medical University (Shanghai, China) in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council, 1996).
1. Animals
- Use Sprague-Dawley (SD) rats of two months (200–250 g). For each behavioral assay, use a separate group of rats. Always use separate control and experimental groups.
NOTE: There were two autonomic tests: EVM and FWR. The EVM had three conditions in addition to a control group (= 4) with three behavioral assays (balance beam, defecation counting and open field = 3) with 8 rats in each for a total of 96 rats (4 x 3 x 8). The FWR had one condition in addition to a control group (= 2) with three behavioral assays (balance beam, defecation counting and open field = 3) with 8 rats in each for a total of 48 rats (2 x 3 x 8). In total, we report 144 rats. - Cage rodents under a constant 25 °C temperature and 60%–70% humidity.
- House rodents in 12 h/12 h light/dark cycles with access to food and drinking water ad libitum.
NOTE: Since the following protocols are behavioral experiments, rats should be handled gently. Handling animals should be with both hands with body and rear support, so as not to induce anxiety. - Perform experiments (EVM and FWR) and evaluation assays (balance beam and open field evaluation) in the darkness to minimize visual cues.
2. Elevator vertical motion device
- Perform the elevator vertical motion procedures in complete darkness to minimize visual cues.
- Place the rodents in the Plexiglas box (22.5 cm x 26 cm x 20 cm). Here the Plexiglas box can accommodate four rodents (custom-made device).
- Ensure the box is fastened shut and securely closed to avoid rodents falling out. Place the Plexiglass box on the elevator pad of the elevator vertical motion device (custom-made device).
- Turn on the elevator vertical motion device to the lowest setting for acclimatization.
- Set the amplitude as 22 cm up and 22 cm down from neutral. Incrementally change elevator vertical motion as follows:
- Set the initial periods as 2,500 ms for 5 min, 2,000 ms for 5 min, and 1,500 ms for 5 min.
- Use a test period of 1000 ms for 2 h.
- Slow the device in reverse using periods of 1500 ms for 5 min, 2000 ms for 5 min, and 2500 ms for 5 min.
3. Ferris-wheel rotation device
- Ferris-wheel rotation device setup
- Place the plexiglass container (22.5 cm x 26 cm x 20 cm) on a wooden bench (custom-made device).
- Place the rodents in the plexiglass container with the long axis of the body perpendicular to the horizontal rotation rod of the Ferris-wheel (custom-made device).
NOTE: The placement with body perpendicular to horizontal rod ensures stimulation of otolith organs (anterior-posterior and vertical direction) during rotation. - Close the plexiglass box securely.
- Place the second set of rodents in the plexiglass container with the long axis of the body perpendicular to the horizontal rotation rod on the second arm of the Ferris-wheel rotation device. Use a second set of rodents with similar mass to balance the Ferris-wheel.
- Securely close the plexiglass box and place on the Ferris-wheel rotation device.
- Ferris-wheel rotation procedure
- Perform the Ferris-wheel rotation procedures in complete darkness to minimize visual cues.
- Start the Ferris-wheel rotating in a clockwise direction at 16°/s2 to reach an angular velocity of 120°/s, and then begin to decelerate at 48°/s2 to reach 0°/s. After a 1 s pause, have the container continue to rotate in a counterclockwise direction in the same manner as above (acceleration at 16°/s2 to reach an angular velocity of 120°/s and then deceleration at 48°/s2 to reach 0°/s). The clockwise-pause-counterclockwise cycle requires approximately 10 s to reach its initial position.
- Continue the clockwise-counterclockwise rotation for 2 h per session for approximately 720 rotations.
4. Evaluation of EVM and FWR
NOTE: The evaluation of Ferris-wheel rotation device and elevator vertical motion is done by three procedures: balance beam testing, defecation counting, and open-field examination. Identical procedures are used to evaluate elevator vertical motion. These evaluation procedures should be done as soon as possible after Ferris-wheel rotation or elevator vertical motion.
- Balance beam
- Balance beam setup
- Set up the balance beam10,11,12 by placing two wooden stools (approximately 0.75 m in height) in the experimental field, approximately 110 cm apart.
- Place a black plastic box (15 cm x 15 cm x 8 cm) on the finish stool.
- Place a narrow wooden beam (2.5 cm x 130 cm) between the two stools, leaving a 100 cm distance between the stool edges, from the start stool to the finish stool.
NOTE: The entrance to the black plastic box should be at the finish line of the 100 cm. - Place a lamp at the start stool. Turn on the lamp.
- Turn off the room lights and ensure that the room is as dark as possible. This ensures the rodent follows the direction of the balance beam from the lighted region to the obscured region.
- Balance beam procedures
NOTE: The motor coordination assay of the balance beam is assessed by measuring the time taken to traverse the elevated wooden beam.- Train each rodent daily for 3 consecutive days, before the examination period, in order to achieve stable performance on the balance beam10. Train by introducing the rat to the beam in the lighted corner and prompting it to cross the beam. Eventually the rat will cross of its own volition. Rats in the present protocol took 3.6 ± 0.9 seconds.
NOTE: Some rodents fail to achieve stable performance during training and should be excluded. Some rodents do not perform the task while others lack motivation to cross the beam. Stable performance was two consecutive trial periods of crossing times less than 4 seconds. If a rat falls off during training or assessment it should be categorized as a rat 'fall' and not assessed further. - For the actual procedure, place the trained rodent on the start stool near the light and simultaneously press start on a stopwatch. The rodent should cross the balance beam rapidly and enter the black box on the finish stool.
- Press start on the stopwatch once the rodent is in place and press stop when the nose enters the dark box on the finish stool. The time to traverse the beam is from start stool to finish stool.
NOTE: Once the rodent is trained, you may perform an intervention or manipulation, such as inducing motion sickness, prior to evaluation. You may also obtain a baseline measurement, prior to the intervention, using the time to traverse of the last training session.
- Train each rodent daily for 3 consecutive days, before the examination period, in order to achieve stable performance on the balance beam10. Train by introducing the rat to the beam in the lighted corner and prompting it to cross the beam. Eventually the rat will cross of its own volition. Rats in the present protocol took 3.6 ± 0.9 seconds.
- Balance beam setup
- Defecation counting
- Place the plexiglass container containing the four rodents on a bench after the Ferris-wheel test period.
- Remove the rodents and place in individual open-field boxes (below).
- Count the number of feces pellets in the plexiglass box attributed to each rodent.
NOTE: A baseline measurement can be obtained, for comparison with the evaluation after elevator motion, by counting feces pellets prior to undergoing elevator vertical motion.
- Open-field examination
- Place the rodents in the open-field box (40 cm x 40 cm x 45 cm).
- Record open field behavior using an IR-video camera for 3 min28,29,30.
- Determine the total distance traveled.
NOTE: It is very important NOT to place the rodent in the open-field box before elevator vertical motion. The environment must be novel to the rodent. Therefore, baseline measurements should NOT be taken for open-field examination.
Representative Results
Figure 2 demonstrates representative balance beam results of time taken to transverse. Rats were trained for 3 consecutive days in order to achieve stable performance on the balance beam10. The subsequent day, rats were evaluated for balance beam performance. In the y-axis of the figure, we have the number of seconds taken for rodents to cross the balance beam for Ferris-wheel, elevator vertical motion, and control groups for demonstrative purposes.
Figure 3 demonstrates representative defecation count results. For elevator vertical motion, rats were in one of three different rotation groups of 0.8 Hz, 0.4 Hz, and 0.2 Hz vertical motion, in addition to a control group, called the static group. The equivalence to our periods of motion is as follows: frequency = 0.8Hz = 1/0.8 = 0.1250s = 1250 ms, frequency = 0.4Hz = 1/0.4 = 0.2500s = 2500 ms, and frequency = 0.2Hz = 1/0.2 = 0.5000s = 5000 ms. The EVM significantly increased defecation (one-way ANOVA, F(3,31) = 20.2306, p < 0.00001). The change in Hz vertical motion increased defecation for 0.4 Hz (t = 3.4064, df = 14, p = 0.0043) and 0.8 Hz (t = 10.6895, df = 14, p < 0.0001). For Ferris-wheel rotation, rats were rotated in a clockwise-pause-counterclockwise cycle lasting approximately 10 s to reach its initial position. The entire session of rotation lasted for 2 h. The Ferris-wheel rotation group was compared to a control group, called the static group. The Ferris-wheel rotation group increased defecation as determined by a t-test (t = 10.6895, df = 14, p < 0.0001).
Figure 4 demonstrates the open field examination of total distance traveled results. These data were collected using commercial video tracking software for the analysis of open field behavior (Table of Materials)28, but several open source software pipelines exist for behavioral video analysis such as Bonsai30 and one our group has developed based on Matlab29. Also, here, the total distance traveled was assessed as a metric, but frame-by-frame differences can be used for determining other behaviors such as vertical motion. For elevator vertical motion, rats were in one of three different rotation groups of 0.8 Hz, 0.4 Hz, and 0.2 Hz vertical motion, in addition to a control group, called the static group. The EVM significantly decreased open field distance traveled (one-way ANOVA, F(3,31) = 16.5994, p < 0.00001). The change in Hz vertical motion decreased open-field locomotion for 0.4 Hz (t = 3.1354, df = 14, p = 0.0073) and 0.8 Hz (t = 5.8929, df = 14, p < 0.001). For Ferris-wheel rotation, rats were rotated in a clockwise-pause-counterclockwise cycle lasting approximately 10 s to reach its initial position. The entire session of rotation lasted for 2 h. The Ferris-wheel rotation group was compared to a control group, called the static group. The Ferris-wheel rotation group decreased open-field locomotion as determined by a t-test (t = 4.3341, df = 14, p = 0.0007).
A number of published studies have employed the protocols described here6,7,8,9,10,11,12. One recent example from our group studied the mechanisms behind anticholingenics mecamylamine and scopolamine alleviating motion sickness-induced gastrointestinal symptoms12.
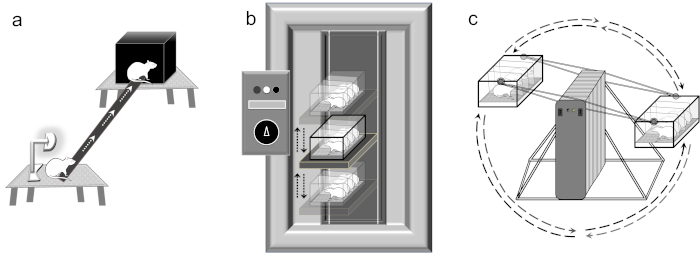
Figure 1: Instrumentation used. (a) Balance Beam. The balance beam is a narrow wooden beam (2.5 cm x 130 cm) between the two stools placed 100 cm (approximately 0.75 m in height) apart. A lamp is placed at the start stool and a black plastic box (15 cm x 15 cm x 8 cm) on the finish stool. (b) Elevator vertical motion device. The elevator vertical motion device amplitude is set at 22 cm up and 22 cm down from neutral. The warm-up vertical motion consists of 2500 ms period for 5 min, 2000 ms for 5 min, and 1500 ms for 5 min. The test motion consists of a 1000 ms period for 2 h. The elevator vertical motion device is slowed in reverse using a 1500 ms period for 5 min, 2000 ms for 5 min, and 2500 ms for 5 min. Rats are placed head towards the front of the elevator vertical motion device. (c) Ferris-wheel rotation device. The Ferris-wheel rotates in a clockwise direction at 16°/s2 accelerating to 120°/s, subsequently decelerating at 48°/s2 to reach 0°/s, pausing for 1 s, and then rotating in a counterclockwise (16°/s2 accelerating to 120°/s, subsequently decelerating at 48°/s2 to reach 0°/s). The clockwise-pause-counterclockwise cycle requires ~10 s to reach its initial position. Rats are placed head towards center of the Ferris-wheel rotation device. Please click here to view a larger version of this figure.
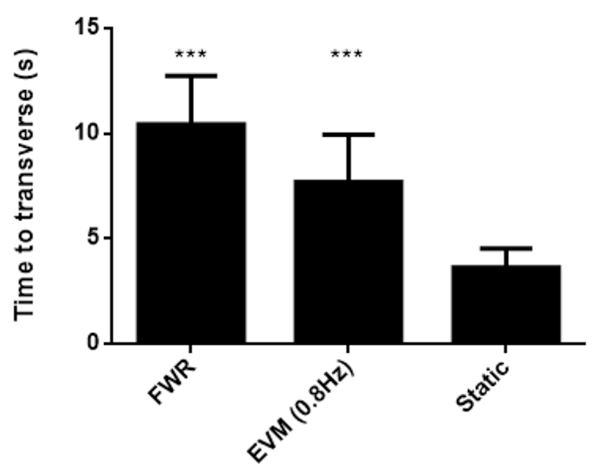
Figure 2: Balance beam results. Time taken to transverse the beam (mean ± standard deviation). The y-axis indicates seconds taken to transverse the beam. Rats were trained for three days prior to evaluation in order to achieve stable performance on the balance beam10. Prior evaluation with the elevator vertical motion or Ferris-wheel devices significantly increases crossing time. Statistical testing was performed by two-tailed t-test with Bonferroni correction between control and every other group. *** indicates p < 0.001. Please click here to view a larger version of this figure.

Figure 3: Defecation count results. Elevator vertical motion results (a) Left panel – Defecation count (mean ± standard deviation) by group for 0.8 Hz, 0.4 Hz, and 0.2 Hz vertical motion, in addition to a control group, called the static group at 0 Hz. Note the significant increase in defecation for 0.8 Hz and 0.4 Hz as indicated by the asterisks. Ferris-wheel rotation results (b) Right panel – Defecation count (mean ± standard deviation) for Ferris-wheel rotation rat group (see description for angular velocity paradigm) and a control group (0 Hz), called the static group. Note the significant increase in defecation for the rotation group as indicated by the asterisks. Please click here to view a larger version of this figure.
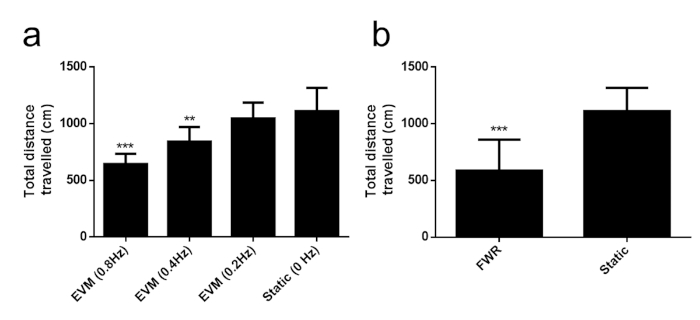
Figure 4: Total distance traveled. (a) Elevator vertical motion results. This panel consists of total distance traveled (mean ± standard deviation) by cm in the open field locomotion test by group for 0.8 Hz, 0.4 Hz, and 0.2 Hz vertical motion, in addition to a control (static) group. Note the significant decrease in total distance traveled for 0.8 Hz and 0.4 Hz as indicated by the asterisks. Statistical testing was performed by two-tailed t-test with Bonferroni correction between control and every other group. ** indicates p < 0.01 and *** indicates p < 0.001. (b) Ferris-wheel rotation results. This panel consists of total distance traveled (mean ± standard deviation) by cm in the open-field locomotion test for Ferris-wheel rotation rat group and a control (static) group. Note the significant decrease in total distance as indicated by the asterisks. Statistical testing was performed by two-tailed t-test between control and Ferris-wheel group. *** indicates p < 0.001. Please click here to view a larger version of this figure.
Discussion
The present study describes assessing autonomic responses to passive motion in rodents using elevator vertical motion and Ferris-wheel rotation. These equipment and procedures can be easily adopted to other rodents and several modifications of the assays exist to confirm vestibular functioning in different circumstances, such as during in pharmacological challenge or surgical interventions. Research in MS elicited by vestibular stimulation has led to the theory that sensory conflict or neuronal mismatch caused by receiving visual information that differs from the anticipated internal model of the environment2,3 leads to autonomic reaction eliciting symptoms such epigastric discomfort, nausea and/or vomiting1. Further theories have outlined that postural instability, as would occur on a yawing ship4,5, elicits autonomic reaction. Despite these significant advances, questions remain that can be aided by evaluation protocols such as elevator vertical motion and Ferris-wheel rotation.
A critical step for balance beam is training. Rats must be motivated and have confidence to cross the beam; otherwise, balance (i.e., vestibular integrity) is not measured in an evaluation period. For researchers interested in examining anxiety14,17 or traumatic injury15,16,17, other behaviors during training or balance beam crossing may be relevant. For example, in anxiety research using the balance beam, defecation, urination, falls, and missteps can be enumerated14. Also in some research areas, rodents that lack motivation to cross the beam may be evaluated differently13,14,15,16,17. It is critical during elevator vertical motion and Ferris-wheel rotation to ensure that the box is fastened shut and securely closed, as rodents in an unsecured box may be propelled and injured. Also, ensure that rodents are evaluated in the open-field box28,29,30 only once and immediately after the elevator vertical motion and Ferris-wheel to ensure rapid evaluation of vestibular effects.
The above-mentioned protocols use quantitative measures. Therefore, the limitations for balance beam include rodents that lack motivation to cross the beam, as balance is the behavior being evaluated. Limitations for the elevator vertical motion and Ferris-wheel rotation defecation assays include requiring a well-fed rodent. This is necessary; otherwise, the rodent may not experience a robust autonomic reaction to vestibular stimulation. It is good practice to observe baseline defecation count for a normal/control period of 2.5 h duration for comparative purposes.
Another important consideration when using the protocols, and interpreting results, is differences in motion sickness responses across species. In humans, and also other species like cats and dogs, retching and vomiting are two common symptoms31,32,33,34. Rats, on the other hand, cannot vomit. However, rats display motion sickness symptoms such as pica35,36, defecation response37, and spontaneous locomotion reduction35,38. Also, humans rely primarily on vision for sensory input and motion sickness is likely related to sensory conflict with the vestibular system2,39. In rats, especially albino rats (e.g., Sprague-Dawley), vision is not typically the primary sense, but rather somatosensory (whiskers). This may lead to inter-species differences in the relative contributions of different sensory inputs to the conflict. Lastly, there are inter-rodent species differences in the motion sickness response. For example, the shrew mouse (Suncus murinus) is able to have an emetic response40,41.
Collectively the procedures described form a short battery of assessments for the examination and evaluation of autonomic reactions in rodents during motion sickness6,7,8,9,10,11. The present techniques coupled to more physiological measures such as electrophysiology to determine the cortical consequences during vestibular stimulation would be of great interest.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by the Hong Kong Research Grants Council, Early Career Scheme, Project #21201217 to C. L. The FWR device has a patent in China: ZL201120231912.1.
Materials
| Elevator vertical motion device | Custom | Custom-made Elevator vertical motion device to desired specifications | |
| Ethovision | Noldus Information Technology | Video tracking software | |
| Ferris-wheel rotation device | Custom | Custom-made Ferris-wheel rotation device to desired specifications | |
| Latex, polyvinyl or nitrile gloves | AMMEX | Use unpowdered gloves 8-mil | |
| Open field box | Custom | Darkened plexiglass box with IR camera | |
| Rat or mouse | JAX labs | Any small rodent | |
| Small rodent cage | Tecniplast | 1284L | |
| Wooden beam and stools | Custom | Custom-made wooden beam and stools to specifications indicated |
Referanslar
- Balaban, C. D. Vestibular autonomic regulation (including motion sickness and the mechanism of vomiting). Current Opinion in Neurology. 12, 29-33 (1999).
- Reason, J. T. Motion sickness adaptation: a neural mismatch model. Journal of the Royal Society of Medicine. 71, 819-829 (1978).
- Keshavarz, B., Hettinger, L. J., Kennedy, R. S., Campos, J. L. Demonstrating the potential for dynamic auditory stimulation to contribute to motion sickness. PLOS One. 9, 101016 (2014).
- Stoffregen, T. A., Chen, F. C., Varlet, M., Alcantara, C., Bardy, B. G. Getting your sea legs. PLoS One. 8, 66949 (2013).
- Smart, L. J., Pagulayan, R. J., Stoffregen, T. A. Self-induced motion sickness in unperturbed stance. Brain Research Bulletin. 47, 449-457 (1998).
- Wang, J. Q., et al. Temporal change in NMDA receptor signaling and GABAA receptor expression in rat caudal vestibular nucleus during motion sickness habituation. Brain Research. 1461, 30-40 (2012).
- Cai, Y. L., et al. Glutamatergic vestibular neurons express FOS after vestibular stimulation and project to the NTS and the PBN in rats. Neuroscience Letters. 417, 132-137 (2007).
- Cai, Y. L., et al. Decreased Fos protein expression in rat caudal vestibular nucleus is associated with motion sickness habituation. Neuroscience Letters. 480, 87-91 (2010).
- Wang, J. Q., Qi, R. R., Zhou, W., Tang, Y. F., Pan, L. L., Cai, Y. Differential Gene Expression profile in the rat caudal vestibular nucleus is associated with individual differences in motion sickness susceptibility. PLoS One. 10, 0124203 (2015).
- Zhou, W., et al. Sex and age differences in motion sickness in rats: The correlation with blood hormone responses and neuronal activation in the vestibular and autonomic nuclei. Frontiers in Aging Neuroscience. 9, 29 (2017).
- Wang, J., Liu, J., Pan, L., Qi, R., Liu, P., Zhou, W., Cai, Y. Storage of passive motion pattern in hippocampal CA1 region depends on CaMKII/CREB signaling pathway in a motion sickness rodent model. Scientific Reports. 7, 43385 (2017).
- Qi, R., et al. Anti-cholinergics mecamylamine and scopolamine alleviate motion sickness-induced gastrointestinal symptoms through both peripheral and central actions. Neuropharmacology. 146, 252-263 (2019).
- Luong, T. N., Carlisle, H. J., Southwell, A., Patterson, P. H. Assessment of motor balance and coordination in mice using the balance beam. Journal of Visualized Experiments. (49), e2376 (2011).
- Kalueff, A. V., Minasyan, A., Tuohimaa, P. Behavioural characterization in rats using the elevated alley Suok test. Behavioural Brain Research. 30 (1), 52-57 (2005).
- Piot-Grosjean, O., Wahl, F., Gobbo, O., Stutzmann, J. M. Assessment of sensorimotor and cognitive deficits induced by a moderate traumatic injury in the right parietal cortex of the rat. Neurobiology of Disease. 8 (6), 1082-1093 (2001).
- Goldstein, L. B., Davis, J. N. Beam-walking in rats: Studies towards developing an animal model of functional recovery after brain injury. Journal of Neuroscience Methods. 31 (2), 101-107 (1990).
- Sweis, B. M., et al. modified beam-walking apparatus for assessment of anxiety in a rodent model of blast traumatic brain injury. Behavioural Brain Research. 296, 149-156 (2016).
- Hess, B. J., Dieringer, N. Spatial organization of the maculo-ocular reflex of the rat: Responses during off-vertical axis rotation. European Journal of Neuroscience. 2, 909-919 (1990).
- Armstrong, P. A., et al. Preserved otolith organ function in caspase-3-deficient mice with impaired horizontal semicircular canal function. Experimental Brain Research. 233 (6), 1825-1835 (2015).
- Riccio, D. C., Thach, J. S. Response suppression produced by vestibular stimulation in the rat. Journal of the Experimental Analysis of Behavior. 11 (4), 479-488 (1968).
- Rabbath, G., et al. Abnormal vestibular control of gaze and posture in a strain of a waltzing rat. Experimental Brain Research. 136, 211-223 (2001).
- Brettler, S. C., et al. The effect of gravity on the horizontal and vertical vestibulo-ocular reflex in the rat. Experimental Brain Research. 132, 434-444 (2000).
- Hutchison, S. L. Taste aversion in albino rats using centrifugal spin as an unconditioned stimulus. Psychological Reports. 33 (2), 467-470 (1973).
- Green, K. F., Lee, D. W. Effects of centrifugal rotation on analgesia and conditioned flavor aversions. Physiology & Behavior. 40 (2), 201-205 (1987).
- Tse, Y. C., et al. Developmental expression of NMDA and AMPA receptor subunits in vestibular nuclear neurons that encode gravity-related horizontal orientations. Journal of Comparative Neurology. 508 (2), 343-364 (2008).
- Lai, C. H., Tse, Y. C., Shum, D. K., Yung, K. K., Chan, Y. S. Fos expression in otolith-related brainstem neurons of postnatal rats following off-vertical axis rotation. Journal of Comparative Neurology. 470 (3), 282-296 (2004).
- Lai, S. K., Lai, C. H., Yung, K. K., Shum, D. K., Chan, Y. S. Maturation of otolith-related brainstem neurons in the detection of vertical linear acceleration in rats. European Journal of Neuroscience. 23 (9), 2431-2446 (2006).
- Aitken, P., Zheng, Y., Smith, P. F. Ethovision analysis of open field behaviour in rats following bilateral vestibular loss. Journal of Vestibular Research. 27 (2-3), 89-101 (2017).
- Gao, V., Vitaterna, M. H., Turek, F. W. Validation of video motion-detection scoring of forced swim test in mice. Journal of Neuroscience Methods. 235, 59-64 (2014).
- Lopes, G., et al. Bonsai: an event-based framework for processing and controlling data streams. Frontiers in Neuroinformatics. 9, 7 (2015).
- Conder, G. A., Sedlacek, H. S., Boucher, J. F., Clemence, R. G. Efficacy and safety of maropitant, a selective neurokinin 1 receptor antagonist, in two randomized clinical trials for prevention of vomiting due to motion sickness in dogs. Journal of Veterinary Pharmacology and Therapeutics. 31, 528-532 (2008).
- Percie du Sert, N., Chu, K. M., Wai, M. K., Rudd, J. A., Andrews, P. L. Telemetry in a motion-sickness model implicates the abdominal vagus in motion-induced gastric dysrhythmia. Experimental Physiology. 95, 768-773 (2010).
- Lackner, J. R. Motion sickness: more than nausea and vomiting. Experimental Brain Research. 232, 2493-2510 (2014).
- Lucot, J. B. Effects of naloxone on motion sickness in cats alone and with broad spectrum antiemetics. Autonomic Neuroscience. 202, 97-101 (2016).
- McCaffrey, R. J. Appropriateness of kaolin consumption as an index of motion sickness in the rat. Physiology & Behavior. 35, 151-156 (1985).
- Horn, C. C., et al. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One. 8 (4), 60537 (2013).
- Ossenkopp, K. -. P., Frisken, N. L. Defecation as an index of motion sickness in the rat. Physiological Psychology. 10, 355-360 (1982).
- Ossenkopp, K. P., Rabi, Y. J., Eckel, L. A., Hargreaves, E. L. Reductions in body temperature and spontaneous activity in rats exposed to horizontal rotation: abolition following chemical labyrinthectomy. Physiology & Behavior. 56, 319-324 (1994).
- Oman, C. M. Motion sickness: a synthesis and evaluation of the sensory conflict theory. Canadian Journal of Physiology and Pharmacology. 68, 294-303 (1990).
- Hu, D. L., et al. Emesis in the shrew mouse (Suncus murinus) induced by peroral and intraperitoneal administration of staphylococcal enterotoxin A. Journal of Food Protection. 62, 1350-1353 (1999).
- Ueno, S., Matsuki, N., Saito, H. Suncus murinus as a new experimental model for motion sickness. Life Sciences. 43, 413-420 (1988).

