Thoracic Spinal Cord Hemisection Surgery and Open-Field Locomotor Assessment in the Rat
Özet
The rat thoracic spinal hemisection is a valuable and reproducible model of unilateral spinal cord injury to investigate the neural mechanisms of locomotor recovery and treatment efficacy. This article includes a detailed step-by-step guide to perform the hemisection procedure and to assess locomotor performance in an open-field arena.
Abstract
Spinal cord injury (SCI) causes disturbances in motor, sensory, and autonomic function below the level of the lesion. Experimental animal models are valuable tools to understand the neural mechanisms involved in locomotor recovery after SCI and to design therapies for clinical populations. There are several experimental SCI models including contusion, compression, and transection injuries that are used in a wide variety of species. A hemisection involves the unilateral transection of the spinal cord and disrupts all ascending and descending tracts on one side only. Spinal hemisection produces a highly selective and reproducible injury in comparison to contusion or compression techniques that is useful for investigating neural plasticity in spared and damaged pathways associated with functional recovery. We present a detailed step-by-step protocol for performing a thoracic hemisection at the T8 vertebral level in the rat that results in an initial paralysis of the hindlimb on the side of the lesion with graded spontaneous recovery of locomotor function over several weeks. We also provide a locomotor scoring protocol to assess functional recovery in the open-field. The locomotor assessment provides a linear recovery profile and can be performed both early and repeatedly after injury in order to accurately screen animals for appropriate time points in which to conduct more specialized behavioral testing. The hemisection technique presented can be readily adapted to other transection models and species, and the locomotor assessment can be used in a variety of SCI and other injury models to score locomotor function.
Introduction
Spinal cord injury (SCI) is associated with severe disturbances in motor, sensory, and autonomic function. Experimental animal models of SCI are valuable tools to understand the anatomical and physiological events involved in SCI pathology, to investigate the neural mechanisms in repair and recovery, and to screen for efficacy and safety of potential therapeutic interventions. The rat is the most commonly used species in SCI research1. Rat models are low cost, easy to reproduce, and a large battery of behavioral tests are available to assess functional outcomes2. Despite some differences in tract locations, the rat spinal cord shares overall similar sensorimotor functions with larger mammals, including primates3,4. Rats also share analogous physiological and behavioral consequences to SCI that relate to humans5. Non-human primate and large animal models can provide a closer approximation of human SCI6 and are essential to prove treatment safety and efficacy prior to human experimentation, but are less commonly used due to ethical and animal welfare considerations, expenses, and regulatory requirements7.
Rat transection SCI models are performed by the targeted interruption of the spinal cord with a selective lesion using a dissection knife or iridectomy scissors after a laminectomy. Compared to a complete transection, partial transection in the rat results in a less severe injury, easier postoperative animal care, spontaneous locomotor recovery, and more closely models SCI in humans which is predominately incomplete with partial sparing of tissue connecting the spinal cord and supraspinal structures8. A unilateral hemisection disrupts all ascending and descending tracts on one side only, and produces quantifiable and highly reproducible locomotor deficits, enhancing exploration of the underlying biological mechanisms. The most prominent functional consequence of the hemisection is an initial limb paralysis on the same side and below the level of the lesion with graded spontaneous recovery of locomotor function over several weeks9,10,11,12. The hemisection model is particularly useful to investigate neural plasticity of damaged and residual tracts and circuits associated with functional recovery9,11,12,13,14,15,16,17,18. Specifically, hemisection performed at the thoracic level, i.e., above the spinal circuits that control hindlimb locomotion, is particularly useful for investigating changes in locomotor control. As a non-linear relationship exists between lesion severity and locomotor recovery after SCI19, appropriate behavioral testing to assess functional outcomes is paramount in experimental models.
A comprehensive battery of behavioral tests are available to assess specific aspects of functional locomotor recovery in the rat2,20. Many locomotor tests do not provide reliable measures early after SCI as rats are too disabled to support their body weight. A measure of spontaneous locomotor performance that is sensitive to deficits early after injury, and does not require preoperative training or specialized equipment, is beneficial in order to monitor locomotor recovery for appropriate time points in which to supplement specialized behavioral testing. The Martinez open-field assessment score10, originally developed for evaluating locomotor performance after cervical SCI in the rat, is a 20-point ordinal score assessing global locomotor performance during spontaneous overground locomotion in an open-field. Scoring is conducted separately for each limb using a rubric that evaluates specific parameters of a range of locomotor measures including articular limb movement, weight support, digit position, stepping abilities, forelimb-hindlimb coordination, and tail position. The assessment score is derived from the Basso, Beattie and Bresnahan (BBB) open-field rating scale designed to evaluate locomotor performance after thoracic contusion21. It is adapted to accurately and reliably evaluate both forelimb and hindlimb locomotor function, allows for independent assessment of the different scoring parameters that is not amenable with the hierarchical scoring of the BBB, and provides a linear recovery profile10. Additionally, in comparison to the BBB, the assessment score is sensitive and reliable in more severe injury models10,11,20,22. The assessment score has been used to assess locomotor impairment in the rat following cervical10,12 and thoracic9 SCI alone and in combination with traumatic brain injury23.
We present here a detailed step-by-step protocol for performing a thoracic hemisection SCI at the T8 vertebral level in the female Long-Evans rat, and for assessing hindlimb locomotor recovery in the open-field.
Protocol
The experiments described in this article were performed in compliance with the guidelines of the Canadian Council on Animal Care and were approved by the ethics committee at the Université de Montréal.
1. Thoracic hemisection surgery
- Wear appropriate protective equipment (gloves, mask, and gown) to maintain an aseptic environment for surgery. Clean the surgical area with alcohol wipes, and place sterile surgical drapes over the surgical field. Sterilize surgical tools and place on the surgical field.
- Anesthetize the rat under a mixture of isoflurane gas (3% induction, 0.5−3% maintenance) and oxygen (1 L/min). Confirm proper surgical anesthetic depth by verifying the absence of toe pinch and corneal reflex responses. Continuously monitor the rat during the entire procedure, and adjust the amount of anesthetic delivery as required to maintain surgical anesthetic depth.
- Shave the dorsal trunk between the hip and the neck, place the rat on the surgical field, disinfect the incision site with alcohol wipes and proviodine solution, and maintain core body temperature at 37 °C using a feedback-controlled heating pad monitored by rectal thermometer.
- Place ophthalmic ointment on the eyes to keep them hydrated and reapply throughout surgery as required.
- Make a 2.5 cm incision in the skin overlaying the T6−T10 vertebrae with a scalpel. Retract the skin and superficial fat using blunt dissection scissors.
NOTE: The T6−T10 vertebral segments can be identified either rostrally by gentle palpation of the dorsal spinal segments from the base of the skull starting from the noticeable protuberance of the 2nd thoracic vertebra24, or caudally by palpation of the most posterior floating rib which will induce movement in the 13th thoracic vertebrae. - Separate the paravertebral muscles inserting on the dorsal aspect of the T7−T9 vertebrae using blunt dissection scissors and a self-retaining retractor. Debride and clear any remaining tissue using fine forceps and cotton tipped applicators to expose the spinous processes and vertebral laminae.
NOTE: This, and the following steps are greatly aided by microscopic visualization (~5−15x). - Carefully cut the facets (zygapophysial joints) bilaterally on the T7 and T8 vertebrae with delicate bone trimmers. Cut the dorsal connective tissue between the T8 and T9 vertebral laminae superficially with a scalpel (1 mm depth) being careful not to injure the underlaying cord.
- Remove the spinous process of the T8 vertebra with bone trimmers. With curved hemostatic forceps carefully clamped on the T7 spinous process, rotate the caudal end of the T8 laminae slightly rostrally (~20°), insert the bone trimmers under the T8 lamina, and make a midline cut extending along the lamina. Continue the laminectomy by repeating the cuts on the left and right side of the vertebral lamina medial to the transverse processes to expose the spinal cord.
NOTE: Be careful to remove all bone fragments created from the laminectomy. - Drip lidocaine (2%, 0.1 mL) in the exposed spinal canal and remove the dura overlaying the T8 spinal segment using fine forceps and iridectomy scissors. Repeat lidocaine administration to the exposed cord and identify the midline of the cord by visualization of a centre line created between the spinous processes extending between exposed T7−T9 vertebra.
NOTE: Along with the spinous processes on T7 and T9, the exposed dorsal root ganglia on T8 can also be used to aid identification of midline and a 30 G needle can be placed in the midline of the cord to aid with the subsequent hemisection. - Hemisect the spinal cord from midline towards the one side with a dissecting knife. Be careful not to cut through the anterior spinal artery on the ventral side (do not apply firm pressure to the vertebral body). Using iridectomy scissors, carefully cut through any remaining tissue on the lesioned side of the spinal cord to ensure the ventrolateral quadrant is appropriately transected.
- Place sterile saline-soaked hemostatic sponge (~6 x 2 mm) in the exposed cavity above the spinal cord and suture the muscle layers (4-0 polyglactin 910). Next, suture the skin around the incision site.
- Provide adequate analgesic (buprenorphine 0.05 mg/kg subcutaneous [s.c.]), antibiotic (enrofloxacin, 10 mg/kg s.c.), and replenish lost fluids with 5 cc lactated ringer’s solution (intraperitoneal [i.p.]) immediately after surgery.
- Remove the rat from anesthesia. Place the rat in a warm environment under a heating pad or lamp (~ 33 °C) until the animal is fully awake.
- Provide supplemental analgesia daily over the first 3 post-surgical days and continually monitor for signs of pain, weight loss, improper micturition, infection, problems with wound healing, or autophagia.
2. Open-field testing procedure and locomotor performance scoring
- Handle rats daily for 1 week and habituate them to the arena for two 5-min sessions prior to testing to acclimatize to being picked up, gently from the mid-trunk, while in the open-field and to ensure measurement reliability during testing.
- Place a camera at ground level facing the circular plexiglass open-field arena to record testing sessions for offline analysis (30−60 frames/s minimum).
- Begin video recording and place the rat in the center of the arena under dim light conditions to encourage locomotor activity.
- Continue the testing session for 4 min to ensure an adequate amount of locomotor bouts for analysis. Pick up and replace rats in the center of the arena when they remain stationary for longer than 20 s to promote locomotion.
- Score locomotor performance of the recorded testing session by completing the rubric provided in Table 1 according to the parameters in the following subsections.
NOTE: It is helpful to score each parameter separately by repeated viewing of the recorded testing session using software that allows for variable playback speed and frame-by-frame analysis (e.g., VLC media player).- For articular limb movements, score hindlimb joint movements during spontaneous locomotion separately for the ankle, knee, and hip as either normal (more than half of the range of motion, awarded score = 2), slight (less than half of the range of motion, awarded score = 1), or absent (awarded score = 0).
- For weight support, evaluate the ability of hindlimb extensor muscles to contract and support loaded body weight when the limb is on the ground separately for when the rat is stationary as well as during active locomotion. Award a score of 1 when weight support is present and a score of 0 when weight support is absent.
NOTE: Stationary weight support is deemed a perquisite for active weight support. - For digit position, evaluate the position of the hindlimb digits while the rat is stationary and during locomotion. Award a score of 2 when hindlimb digits are extended, spaced apart from one another, and tonic during locomotion in more than 50% of the testing period (considered normal). Award a score of 1 when digits remain predominantly flexed and a score of 0 when digits remain predominantly atonic.
- For stepping, complete this parameter only if the rat can support its body weight during stepping. Evaluate stepping by rating the orientation of hindlimb paw placement at the time of initial contact and at lift off from the ground in addition to the fluidity of the swing phase during stepping.
NOTE: There are 3 scores for this parameter described in the following subsections separately evaluating: 1) the axial orientation of paw placement at limb contact (dorsal/plantar placement), 2) the longitudinal orientation of paw placement at initial contact and during lift (parallel to the body axis or rotated internally/externally), and 3) the quality of limb movement during swing (regular or irregular).- For the paw placement at limb contact, score the axial orientation of the paw placement at limb contact as 0 when dorsal placements occur in more than 50% of steps.
NOTE: Plantar placement is deemed a perquisite for scoring the orientation of paw at contact and lift (step 2.5.4.2), swing movement (step 2.5.4.3) and forelimb-hindlimb coordination (step 2.5.5). - For the paw orientation at limb contact and lift, award a score of 2 when the longitudinal paw and body axes are parallel and a score of 1 when the limb is rotated externally or internally, separately for both limb contact and lift.
- For the swing movement, award a score of 2 when hindlimb joints move in a harmonious and regular way during swing and a score of 1 when jerky or spasmodic movements of the joints occur during swing.
- For the paw placement at limb contact, score the axial orientation of the paw placement at limb contact as 0 when dorsal placements occur in more than 50% of steps.
- For forelimb-hindlimb coordination, complete this parameter only if 4 consecutive steps occur during testing and if the limbs can actively support body weight. Award a score of 3 when coordination is consistent (>90% of steps), 2 when frequent (50−90% of steps), 1 when occasional (<50% of steps), or 0 when absent (0% of steps).
NOTE: Forelimb-hindlimb coordination is defined as a regular alternation in stepping between the hindlimb being scored and the forelimb on the same side of the body. - For tail position, evaluate the tail position during locomotion as either up (off the ground, awarded score = 1) or down (touching the ground, awarded score = 0).
NOTE: An elevated tail position during locomotion is an indicator of trunk stability in the rat. After hemisection, the tail is normally held close to or touching the ground as trunk stability is impaired. - Add the individual scores from each parameter to provide a total for each hindlimb of a maximum of 20 points.
NOTE: A score of 20 indicates normal locomotor performance. Scores <20 represent increasing amounts of locomotor impairment and a score of 0 indicates limb paralysis.
Representative Results
Reproducible lesions with a high degree of consistency can be generated with the hemisection technique. To assess and compare lesions sizes between experimental groups, the maximal area of the lesion as a percentage of the total cross-section of the spinal cord can be readily calculated with histological staining of spinal cord sections. Figure 1 shows a representative lesion of the left hemicord and an overlay of the proportion of maximal lesion area shared between rats with a mean lesion size of 47.3% ± 4.0% of the cross-sectional cord area (n = 6).
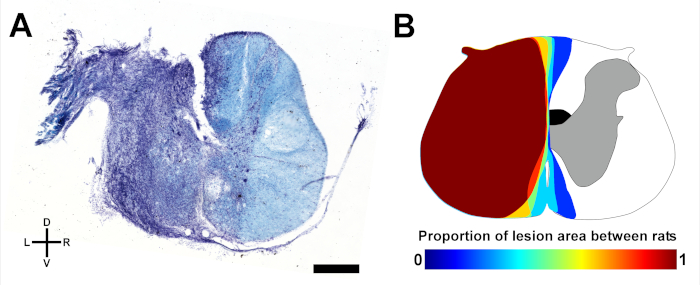
Figure 1: Representative spinal lesions. (A) Microphotograph of a coronal spinal section at the lesion epicenter from a hemisected rat stained with cresyl violet (cell bodies, purple) and luxol fast blue (myelin, blue) indicating damage to the grey and white matter concentrated in the left hemicord. D, dorsal; V, ventral; L, left; R, right. Scale bar: 1 mm. (B) Schematic overlay of the shared proportion of maximal lesion area in a group of rats (n = 6). The location of the crossed corticospinal tract in the dorsal funiculus on the right side is shaded in black. Please click here to view a larger version of this figure.
The primary consequence of the hemisection is an initial paralysis of the hindlimb on the side of the lesion during the first two to three postoperative days. Locomotor performance of the more affected hindlimb improves rapidly in the rat after hemisection over the first few weeks after injury. Small deficits in the opposite hindlimb are commonly observed initially after the hemisection that can reflect compensation for the more affected limb, or deficits resulting from a lack of postural stability, weight support, and consistent stepping. A large and persisting deficit in the opposite hindlimb would indicate a bilateral lesion extending into the opposing hemicord.
A sample locomotor performance scoring rubric is provided in Table 1.
Table 1: Sample scoring sheet. Sample locomotor performance scoring rubric. For each parameter, the possible scores are indicated in parentheses. I, internal; E, external; P, parallel; FL-HL, forelimb-hindlimb. Please click here to download this file.
The time course of representative changes in locomotor performance in the intact state and over the first five weeks after a left side hemisection in separate groups of rats (n = 6 per group) is depicted in Figure 2.
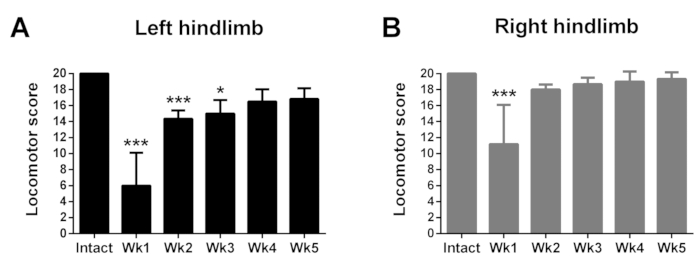
Figure 2: Representative time course of changes in hindlimb locomotor performance in the open-field in the intact state and for five weeks after a left side thoracic hemisection. Performance of the left hindlimb (A) is significantly impaired from intact values during the first three weeks after hemisection, and of the right hindlimb (B) during the first week after hemisection. Data are plotted as group mean ± standard deviation (SD; n = 6 per group). Statistical analyses were performed with Kruskal-Wallis non-parametric tests supplemented with Dunn’s multiple comparison tests to assess group differences between time points. *p < 0.05, ***p < 0.001. Please click here to view a larger version of this figure.
Discussion
A major strength of the hemisection technique is the selectivity and reproducibility of the lesion which leads to reduced variability in histological and behavioral phenotypes between animals25. In order to ensure a unilateral lesion at the appropriate spinal level, accurate identification of both the proper vertebral segment and spinal cord midline is critical. As there can be a tendency for the spinal cord to rotate in the direction of the cut during the hemisection procedure, it can be beneficial to stabilize the cord delicately with fine forceps placed on either side during the procedure. Placing the rat in a stereotaxic frame with the tail gently taped under light tension can help with stability and proper vertebral alignment during the procedure. A spinal clamp attached to the stereotaxic frame and a spinous process can also be used to enhance stability of the vertebral column, but we find that its presence can restrict access to the cord with surgical tools and requires awkward approach angles during the surgery. It is also essential to remove any bone fragments left in the spinal canal from the laminectomy as they can cause unwanted compression injury to the cord and promote secondary damage.
Rats should be constantly observed during the surgery to monitor necessary vital signs such as core temperature and breathing, as hypothermia is a leading cause of mortality both during anesthesia administration and initially after surgery. Regulation of core body temperature with a rectal probe and feedback-controlled heating pad can greatly avoid temperature complications. A pulse oximeter can also be used to monitor blood oxygenation and heart rate to regulate anesthetic depth. We find that fluid replenishment immediately after surgery with lactate ringer’s solution warmed to body temperature results in a more rapid recovery time for the rat to awaken after surgery, regain autonomic control of body temperature, and be able to drink and eat.
Post-surgical monitoring of the rat is essential after the hemisection surgery, especially for signs of improper micturition, pain, infection, weight loss, problems with wound healing, or autophagia. Consultation with veterinary staff for evaluation and treatment is crucial in situations of post-surgical complications. In particular, acute spinal shock or unintended bilateral lesions may interfere with micturition that can lead to potentially fatal infections. Carefully monitor the bladder of the rat after surgery and manually void three times per day if full by gentle pressure from the ventral side of the bladder descending caudally. We use female Long-Evans rats as they have a significantly shorter and straighter urethra than males that leads to a more rapid onset of an automatic urinary bladder, easier micturition, and lower rates of urinary tract infections2. Weights should also be monitored and a loss >20% from baseline warrants investigation into food and water intake. The teeth should be checked for malocclusion, the abdomen for ileus, and rats given appropriate supplementary fluids and nutrition such as hydrogel or a liquid diet. A cyst may rarely form under the incision site that can be drained safely with a syringe without complication in consultation with veterinary staff.
The Martinez open-field locomotor assessment procedure provides a simple technique that does not require any specialized equipment, preoperative training, or food deprivation of the animal to perform. The assessment can be performed as early as the animal recovers from anesthesia and can be used to screen animals for appropriate recovery indices (e.g., recovery of body weight support) when more rigorous and specific locomotor testing can be supplemented such as automated gait assessment of overground locomotion26,27,28, kinematic analyses during treadmill locomotion29,30,31,32, grid walking33, and ladder rung walking9,34. Importantly, while the BBB scale has been shown to not be linear with locomotor recovery as scores tend to cluster around certain values19, the Martinez open-field locomotor assessment provides a linear scoring profile during the recovery process10. To ensure reliable behavioral data, it is important to minimize the number of confounders during testing and analysis. To help reduce variability during testing, sessions should occur at the same time of day, in the same room, and by the same experimenter. The open-field assessment can be reliably performed over repeated sessions9,10,11,12,23, but rats may become habituated to the environment over time and reduce their activity during testing resulting in an inadequate amount of locomotor bouts for analysis. To overcome immobility during testing, rats that remain stationary for longer than 20 seconds are picked up and replaced in the center of the arena to promote locomotion. Additionally, including a conspecific in the arena during testing that is marked for identification can help promote locomotor activity in the test rat. To ensure reliability in locomotor scoring two raters, preferably blinded, should conduct the analyses as previously described10.
In conclusion, we describe methods for conducting a thoracic spinal cord hemisection in the rat and assessing spontaneous hindlimb locomotor performance in an open-field arena. Although a procedure for conducting lateral hemisections was described, the technique can be readily adapted to perform either dorsal hemisections35, staggered alternating hemisections36,37, or full transections38 depending on the desired lesion location and amount of spared descending supraspinal innervation. Importantly, the technique can also be used in larger animal models, including cats39,40,41 and non-human primates6,42 with comparable deficits observed between small and large animals, making it useful for investigating both the neurobiological mechanisms of recovery and for preclinical therapeutic testing.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Canadian Institutes for Health Research (CIHR; MOP-142288) to M.M. M.M. was supported by a salary award from Fonds de Recherche Québec Santé (FRQS), and A.R.B was supported by a fellowship from FRQS.
Materials
| Baytril | CDMV | 11242 | |
| Blunt dissection scissors | World Precision Instruments | 503669 | |
| Buprenorphine hydrochoride | CDMV | ||
| Camera lens | Pentax | C31204TH | 12.5-75mm, f1.8, 2/3" format, C-mount |
| CMOS video camera | Basler | acA2000-165uc | 2/3" format, 2048 x 1088 pixels, up to 165 fps, C-mount, USB3 |
| Compressed oxygen gas | Praxair | ||
| Cotton tipped applicators | CDMV | 108703 | |
| Delicate bone trimmers | Fine Science Tools | 16109-14 | |
| Dissecting knife | Fine Science Tools | 10055-12 | |
| Dumont fine forceps (#5) | Fine Science Tools | 11254-20 | |
| Ethicon Vicryl 4/0 Violet Braided FS-2 suture (J392H) | CDMV | 111689 | |
| Feedback-controlled heating pad | Harvard Apparatus | 55-7020 | |
| Female Long-Evans rats | Charles River Laboratories | Strain code: 006 | 225-250g |
| Gelfoam | CDMV | 102348 | |
| Curved hemostat forceps | Fine Science Tools | 13003-10 | |
| Hot bead sterilizer | Fine Science Tools | 18000-45 | |
| Hydrogel | 70-01-5022 | Clear H20 | |
| Isofluorane | CDMV | 118740 | |
| Lactated Ringer's solution | CDMV | 116373 | |
| Lidocaine (2%) | CDMV | 123684 | |
| Needle 30 ga | CDMV | 4799 | |
| Open-field area | Custom | Circular Plexiglas arena 96 cm diameter, 40 cm wall height | |
| Opthalmic ointment | CDMV | 110704 | |
| Personal computer | With USB3 connectivity to record video with the listed camera | ||
| Physiological saline | CDMV | 1399 | |
| Proviodine | CDMV | 4568 | |
| Rodent Liquid Diet | Bioserv | F1268 | |
| Scalpal blade #11 | CDMV | 6671 | |
| Self-retaining retractor | World Precision Instruments | 14240 | |
| Vannas iridectomy spring scissors | Fine Science Tools | 15002-08 | |
| Veterinary Anesthesia Machine and isofluarane vaporizer | Dispomed | 975-0510-000 | |
| VLC media player | VideoLAN | videolan.org/vlc |
Referanslar
- Sharif-Alhoseini, M., et al. Animal models of spinal cord injury: a systematic review. Spinal Cord. 55 (8), 714-721 (2017).
- Sedy, J., Urdzikova, L., Jendelova, P., Sykova, E. Methods for behavioral testing of spinal cord injured rats. Neuroscience and Biobehavioral Reviews. 32 (3), 550-580 (2008).
- Butler, A. B., Hodos, W. . Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. , 139-152 (2005).
- Nudo, R. J., Masterton, R. B. Descending pathways to the spinal cord: a comparative study of 22 mammals. Journal of Comparative Neurology. 277 (1), 53-79 (1988).
- Metz, G. A., et al. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. Journal of Neurotrauma. 17 (1), 1-17 (2000).
- Friedli, L., et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Science Translational Medicine. 7 (302), 302ra134 (2015).
- Talac, R., et al. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 25 (9), 1505-1510 (2004).
- Kwon, B. K., Oxland, T. R., Tetzlaff, W. Animal models used in spinal cord regeneration research. Spine. 27 (14), 1504-1510 (2002).
- Brown, A. R., Martinez, M. Ipsilesional motor cortex plasticity participates in spontaneous hindlimb recovery after lateral hemisection of the thoracic spinal cord in the rat. Journal of Neuroscience. 38 (46), 9977-9988 (2018).
- Martinez, M., Brezun, J. M., Bonnier, L., Xerri, C. A new rating scale for open-field evaluation of behavioral recovery after cervical spinal cord injury in rats. Journal of Neurotrauma. 26 (7), 1043-1053 (2009).
- Martinez, M., Brezun, J. M., Zennou-Azogui, Y., Baril, N., Xerri, C. Sensorimotor training promotes functional recovery and somatosensory cortical map reactivation following cervical spinal cord injury. European Journal of Neuroscience. 30 (12), 2356-2367 (2009).
- Martinez, M., et al. Differential tactile and motor recovery and cortical map alteration after C4-C5 spinal hemisection. Experimental Neurology. 221 (1), 186-197 (2010).
- Leszczynska, A. N., Majczynski, H., Wilczynski, G. M., Slawinska, U., Cabaj, A. M. Thoracic hemisection in rats results in initial recovery followed by a late decrement in locomotor movements, with changes in coordination correlated with serotonergic innervation of the ventral horn. PLoS One. 10 (11), e0143602 (2015).
- Ballermann, M., Fouad, K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. European Journal of Neuroscience. 23 (8), 1988-1996 (2006).
- Garcia-Alias, G., et al. Chondroitinase ABC combined with neurotrophin NT-3 secretion and NR2D expression promotes axonal plasticity and functional recovery in rats with lateral hemisection of the spinal cord. Journal of Neuroscience. 31 (49), 17788-17799 (2011).
- Petrosyan, H. A., et al. Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. Journal of Neuroscience. 33 (9), 4032-4043 (2013).
- Schnell, L., et al. Combined delivery of Nogo-A antibody, neurotrophin-3 and the NMDA-NR2d subunit establishes a functional ‘detour’ in the hemisected spinal cord. The European journal of neuroscience. 34 (8), 1256-1267 (2011).
- Shah, P. K., et al. Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain. 136, 3362-3377 (2013).
- Schucht, P., Raineteau, O., Schwab, M. E., Fouad, K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Experimental Neurology. 176 (1), 143-153 (2002).
- Metz, G. A., Merkler, D., Dietz, V., Schwab, M. E., Fouad, K. Efficient testing of motor function in spinal cord injured rats. Brain Research. 883 (2), 165-177 (2000).
- Basso, D. M., Beattie, M. S., Bresnahan, J. C. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of Neurotrauma. 12 (1), 1-21 (1995).
- Barros Filho, T. E. P. d., Molina, A. E. I. S. Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats. Clinics (Sao Paulo, Brazil). 63 (1), 103-108 (2008).
- Inoue, T., et al. Combined SCI and TBI: recovery of forelimb function after unilateral cervical spinal cord injury (SCI) is retarded by contralateral traumatic brain injury (TBI), and ipsilateral TBI balances the effects of SCI on paw placement. Experimental Neurology. 248, 136-147 (2013).
- Vichaya, E. G., Baumbauer, K. M., Carcoba, L. M., Grau, J. W., Meagher, M. W. Spinal glia modulate both adaptive and pathological processes. Brain, Behavior, and Immunity. 23 (7), 969-976 (2009).
- Ahmed, R. U., Alam, M., Zheng, Y. -. P. Experimental spinal cord injury and behavioral tests in laboratory rats. Heliyon. 5 (3), e01324 (2019).
- Ham, T. R., et al. Automated gait analysis detects improvements after intracellular sigma peptide administration in a rat hemisection model of spinal cord injury. annals of biomedical engineering. 47 (3), 744-753 (2019).
- Hamers, F. P. T., Koopmans, G. C., Joosten, E. A. J. CatWalk-assisted gait analysis in the assessment of spinal cord injury. Journal of Neurotrauma. 23 (3-4), 537-548 (2006).
- Neckel, N. D., Dai, H. N., Burns, M. P. A novel multidimensional analysis of rodent gait reveals the compensation strategies employed during spontaneous recovery from spinal cord and traumatic brain injury. Journal of Neurotrauma. , (2018).
- Fouad, K., Metz, G. A. S., Merkler, D., Dietz, V., Schwab, M. E. Treadmill training in incomplete spinal cord injured rats. Behavioural Brain Research. 115 (1), 107-113 (2000).
- Thibaudier, Y., et al. Interlimb coordination during tied-belt and transverse split-belt locomotion before and after an incomplete spinal cord injury. Journal of Neurotrauma. 34 (9), 1751-1765 (2017).
- Alluin, O., et al. Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. Journal of Neurotrauma. 28 (9), 1963-1981 (2011).
- Martinez, M., Delivet-Mongrain, H., Leblond, H., Rossignol, S. Effect of locomotor training in completely spinalized cats previously submitted to a spinal hemisection. Journal of Neuroscience. 32 (32), 10961-10970 (2012).
- Behrmann, D. L., Bresnahan, J. C., Beattie, M. S., Shah, B. R. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. Journal of Neurotrauma. 9 (3), 197-217 (1992).
- Soblosky, J. S., Colgin, L. L., Chorney-Lane, D., Davidson, J. F., Carey, M. E. Ladder beam and camera video recording system for evaluating forelimb and hindlimb deficits after sensorimotor cortex injury in rats. Journal of Neuroscience Methods. 78 (1-2), 75-83 (1997).
- Bareyre, F. M., et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nature Neuroscience. 7 (3), 269-277 (2004).
- Courtine, G., et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nature Medicine. 14 (1), 69-74 (2008).
- van den Brand, R., et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 336 (6085), 1182-1185 (2012).
- Lukovic, D., et al. Complete rat spinal cord transection as a faithful model of spinal cord injury for translational cell transplantation. Scientific Reports. 5, 9640-9640 (2015).
- Wilson, S., et al. The hemisection approach in large animal models of spinal cord injury: overview of methods and applications. Journal of Investigative Surgery. 10, 1-12 (2018).
- Martinez, M., Delivet-Mongrain, H., Leblond, H., Rossignol, S. Incomplete spinal cord injury promotes durable functional changes within the spinal locomotor circuitry. Journal of Neurophysiology. 108 (1), 124-134 (2012).
- Martinez, M., Delivet-Mongrain, H., Leblond, H., Rossignol, S. Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry. Journal of Neurophysiology. 106 (4), 1969-1984 (2011).
- Capogrosso, M., et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 539, 284-288 (2016).

