Formation of Covalent DNA Adducts by Enzymatically Activated Carcinogens and Drugs In Vitro and Their Determination by 32P-postlabeling
Özet
Evaluating the potency of environmental chemicals and drugs, to be enzymatically bioactivated to intermediates generating covalent DNA adducts, is an important field in the development of cancer and its treatment. Methods are described for compound activation to form DNA adducts, as well as techniques for their detection and quantification.
Abstract
Covalent DNA adducts formed by chemicals or drugs with carcinogenic potency are judged as one of the most important factors in the initiation phase of carcinogenic processes. This covalent binding, which is considered the cause of tumorigenesis, is now evaluated as a central dogma of chemical carcinogenesis. Here, methods are described employing the reactions catalyzed by cytochrome P450 and additional biotransformation enzymes to investigate the potency of chemicals or drugs for their activation to metabolites forming these DNA adducts. Procedures are presented describing the isolation of cellular fractions possessing biotransformation enzymes (microsomal and cytosolic samples with cytochromes P450 or other biotransformation enzymes, i.e., peroxidases, NADPH:cytochrome P450 oxidoreductase, NAD(P)H:quinone oxidoreductase, or xanthine oxidase). Furthermore, methods are described that can be used for the metabolic activation of analyzed chemicals by these enzymes as well as those for isolation of DNA. Further, the appropriate methods capable of detecting and quantifying chemical/drug-derived DNA adducts, i.e., different modifications of the 32P-postlabeling technique and employment of radioactive-labeled analyzed chemicals, are shown in detail.
Introduction
The metabolism of xenobiotics (environmental chemicals or drugs) occurs in two phases1. Phases I and II aim to render the originally hydrophobic (not water-soluble) compounds more hydrophilic (water-soluble), thus making them readily excretable via urine, feces or sweat. Phase I (functionalization) reactions include oxidation, reduction, and hydroxylation catalyzed by enzymes such as cytochrome P450s (P450s, CYPs), peroxidases (i.e., cyclooxygenase, COX), aldo-keto reductases (AKRs), and microsomal flavin-containing monooxygenases (FMOs). Phase I also includes reduction reactions, mediated by a variety of reductases i.e., microsomal NADPH:cytochrome P450 reductase (POR) and cytosolic NAD(P)H:quinone oxidoreductase (NQO1), xanthine oxidase (XO), and aldehyde oxidase (AO)1. In the second phase (conjugation), the functional groups that were attached in phase I are used to conjugate small polar molecules to further increase the polarity. Examples of enzymes considered to participate in the reaction of phase II include sulfotransferases (SULTs), N,O-acetyltransferases (NATs), methyltransferases such as catechol-O-methyltransferase (COMT), glutathione S-transferases (GSTs), and uridine diphosphate glucuronosyltransferases (UGTs)1. The classification of enzymes in phase I or II is, however, not rigid, and some enzymes can arguably be grouped into either category.
P450 enzymes (EC 1.14.14.1) are the heme containing proteins present in various organisms, which participate in the biotransformation of many chemicals, catalyzing their conversion3,4. The P450 enzymes catalyze hydroxylation of many substrates, with a reaction where one atom of dioxygen is introduced into the molecule of xenobiotics, while the second atom of oxygen is reduced to form water by the reaction that requires two electrons [the equation (1)]3,4:
RH + O2 + NADPH + H+ → ROH + H2O + NADP+ (1)
P450 enzymes localized in the endoplasmic reticulum membrane of mammalian cells (microsomal P450 systems) are members of the multienzyme monooxygenase system, which further contains NADPH:cytochrome P450 reductase (POR) and cytochrome b5, the substrate of the enzyme termed as NADH:cytochrome b5 reductase. A generally accepted theory hypothesizes that the donor of the two electrons needed for P450 is the NADPH/POR system. Nevertheless, cytochrome b5 might also act as a donor of electrons for P450, namely as a donor of the electron reducing P450 during second reduction of its reaction cycle, where it acts together with NADH:cytochrome b5 reductase2,3,4.
Mammals utilize various P450 enzymes (e.g., the enzymes of families 5, 8, 11, 17, 19, 21, 24, 26, and 27) for the synthesis of valuable endogenous compounds, such as steroids, and use them for catabolism of natural products2,3. The other CYP mammalian enzymes, such as human CYP1A2, 2C9, 2C19, 2D6, and 3A4, metabolize exogenous chemicals that are used as drugs.5,6 The most important enzymes catalyzing metabolism of drugs are CYPs of the 3A subfamily, especially CYP3A4. The conversions of xenobiotics, such as pro-carcinogens and pro-toxicants, are mediated by human CYP1A1, 1A2, 1B1, 2A6, 2E1, and 3A42,5. Most of these CYPs are present in the liver (except CYP1A1 and 1B1). Nevertheless, the CYPs are also expressed in several extrahepatic organs. Such P450s might be of great significance, predominantly when participate they in bioactivation metabolism of chemicals (drugs) to reactive intermediates in these organs7. Various P450s are induced by several compounds that are their substrates, though this is not necessarily the case.
Many P450 enzymes play a role in chemical (drug) toxicity. They can convert the xenobiotics not only into their detoxification metabolites, but also activate them to reactive species, which modify endogenous macromolecules that additionally exhibit different biological properties, usually causing their toxicity. DNA, lipids, and proteins might be the targets for their modification by reactive electrophiles and radicals generated from activated chemicals. In the case of DNA, resolving several important gene responses and their mechanisms are already known2,3,4,5.
The changes in DNA can result in a decrease in cell growth control, and this phenomenon is considered to be the predominant factor leading to development of carcinogenic processes. The generation of covalent DNA adducts with chemicals having carcinogenic potency is judged as one of the most important steps in the initiation phase of carcinogenic processes8,9,10,11. It was demonstrated that relationships between the formation of DNA adducts and tumorigenesis occur, whereas a decrease in the amount of DNA adducts is responsible for chemoprevention8,9,10,11,12,13,14. The formation of carcinogen/drug-derived DNA adducts depends on individual bases of DNA, and is affected by the sequences of these bases in DNA. The repairs of DNA adducts are dependent on their location (on the transcribed or non-transcribed DNA strand) and types of modified nucleotide sequences8,11,12,15,16.
In this article, we describe procedures utilizing the enzyme-catalyzed conversion of chemicals (drugs) to investigate their potency to be activated into metabolites which modified DNA (generating DNA adducts). For covalent DNA binding, the test compound should usually be activated either by oxidative or reductive reactions, depending on individual drugs. Oxidative or reductive activation of tested chemicals is mediated by a P450-dependent enzymatic system present in the microsomal subcellular fraction or by reduction with reductases present both in microsomes (POR, NADH:cytochrome b5 reductase, P450 enzymes) and in cellular cytosolic subcellular fractions (NQO1, XO, AO, peroxidase). Reactive metabolites thereafter bind to DNA forming DNA adducts. Because both oxidative and reductive reactions are important to activate several drugs to these reactive species, the experimental procedures employing the oxidation/reduction enzymatic system are described. Furthermore, the appropriate methods capable of detecting and quantifying these DNA adducts are described in detail.
Two independent procedures to determine whether the test chemical, activated by enzymatic systems, is bound to DNA are recommended: the 32P-postlabeling technique and utilizing radioactive-labeled compound (e.g., 3H or 14C). For the first pilot, screening the 32P-postlabeling assay is recommended. The determination of the DNA content in solutions, precisely evaluated, must precede both methods.
The 32P-postlabeling technique utilizes the enzymatic hydrolysis of DNA modified by non-radioactive chemicals (carcinogen/drug) to 3´-phosphodeoxynucleosides, additional phosphorylation with radioactive phosphorus (32P) at the 5´-OH position, and the separation of chemical-deoxynucleotide adducts from normal (unmodified) deoxynucleotides by chromatography17 (Figure 1). DNA modified by the chemical compound is hydrolyzed by a mixture of endonuclease, micrococcal nuclease, and exonuclease, known as spleen phosphodiesterase. The mixture of hydrolyzed DNA containing both normal (unmodified) and modified deoxyribonucleoside 3´-monophosphates is reacted with [γ-32P]ATP in the presence of carrier (non-radioactive) ATP and T4-polynucleotide kinase at pH 9.5 to form 5´-32P-labeled 3´,5´-bisphosphates ("standard" procedure in Figure 1). The used alkaline pH is capable of minimizing the enzyme activity of T4-polynucleotide kinase to dephosphorylate deoxyribonucleoside 3´-monophosphates at position 3´. Separation and resolution of 32P-labeled adducts from labeled deoxynucleotides that are not modified by chemicals is carried out by multidirectional anion-exchange thin layer chromatography (TLC) on polyethyleneimine (PEI) cellulose (Figure 2). In the first and second elution steps (in D1 and D2 direction), labeled normal (unmodified) deoxynucleotides as well as [32P]phosphate are eluted from the start of the TLC-PEI-cellulose plate using water solutions of electrolyte onto a short piece of chromatographic paper applied on the top of the TLC plate, whereas the deoxynucleotides containing bound chemicals exhibiting hydrophobic properties (carcinogen/drug-adducts) are maintained at the start of PEI-cellulose plate to be additionally resolved with several different solvent systems in D3 and D4 directions (Figure 2). Localization of adducts is performed using screen enhanced autoradiography; the separated adducts are detected as dark recognizable spots on X-ray films. The areas of spots are excised from the plate and used to quantify radioactivity by liquid scintillation or Cerenkov counting. A storage phosphor imaging method that has been adapted to map and quantify DNA adducts on chromatograms detected by the 32P-postlabeling assay is now also used.18 The Instant Imager machine is frequently utilized for such detection and quantification of DNA adducts. This method provides more than 10-times higher sensitivity for detecting 32P than the technique of screen enhanced autoradiography19.
Amounts of DNA adducts are determined as values of relative adduct labeling (RAL), calculated using the equation (2) as follows:
cpm. in adduct deoxynucleotides
RAL = —————————————————————————————– (2)
specific activity of 32P-ATP (in cpm./pmol) x pmol deoxynucleotides
The values of RALs are the ratio of count rates of adducted deoxynucleotides over count rates of total [adducted and normal (unmodified) deoxynucleotides] deoxynucleotides20,21. However, this calculation is based on equal labeling efficiencies of adducts and normal deoxynucleotides22. The classical ("standard") procedure of the 32P-postlabeling technique is appropriate for various DNA adducts (bulky and/or non-bulky adducts), however, its sensitivity is not satisfactory to detect adducts found in low amounts in DNA. Using this procedure, the amount of an adduct in 107 unmodified deoxynucleotides in DNA (0.3 fmol adduct/µg DNA) is detectable.
A variety of modifications of this classical 32P-postlabeling procedure have been utilized to elevate the sensitivity of the technique. Up to 10- to 100-times higher sensitivity of determination of adducts by 32P-labeling has been achieved using limiting levels of [γ-32P]ATP (the intensification procedure).23,24 A further procedure providing an increase in sensitivity of the 32P-postlabeling method utilizes an incubation of digested DNA containing adducts with nuclease P1 (from Penicillium citrinum)21 (Figure 1). This enzyme prefers to dephosphorylate unmodified deoxyribonucleoside 3´-monophosphates, whereas the deoxynucleotides with bound chemicals (adducted nucleotides) are essentially not the substrates of this enzyme. Therefore, dephosphorylated deoxyribonucleoside 3´-monophosphates (i.e., deoxyribonucleosides) are not phosphorylated by T4-polynucleotide kinase by [32P]phosphate from γ-32P]ATP. However, some of nucleotides where chemicals are bound (adducted deoxynucleotides), such as arylamine adducts substituted at C8 of deoxyguanosine, can bedephosphorylated by this enzyme. In contrast, most other adducts (e.g., adducts substituted at N2 of deoxyguanosine) are not dephosphorylated by nuclease P1. This modification of 32P-postlabeling makes this method considerably more sensitive, increasing its sensitivity by more than three orders of magnitude. Moreover, this version of 32P-postlabeling provides a method where higher amounts of DNA (5-10 µg) and an excess of carrier-free [γ- 32P] ATP can be utilized.
Another method to enrich the adducts, described by Gupta25, utilizes the physicochemical properties of bulky deoxynucleotide adducts, which can be extracted into n-butanol in the presence of a phase transfer agent tetrabutylammonium chloride (TBA) (Figure 1) prior to [32P]phosphate labeling, whereas unmodified deoxynucleotides are poorly extracted by this organic solvent. However, less hydrophobic adducts, consisting for example of deoxynucleotides modified with non-aromatic bulky moieties or small alkyl residues, are not effectively extracted with n-butanol. Hence, they are essentially undetectable when are analyzed by this modification of the 32P-postlabeling method.
Both the previously mentioned versions of 32P-postlabeling increase the sensitivity and quantification of DNA adducts enormously (up to three orders of magnitude), being able to detect one adduct per 109,10 normal nucleotides (0.3 – 3 amol/µg DNA). These two methods are recommended for testing the chemicals for their efficiency to covalently bind to DNA and, therefore, they are described in this work in details.
Protocol
All animal experiments were performed in accordance with the Regulations for the Care and Use of Laboratory Animals (311/1997, Ministry of Agriculture, Czech Republic), which is in compliance with the Declaration of Helsinki.
1. Isolation of Hepatic Microsomal and Cytosolic Fractions
- Prepare liver subcellular fractions (microsomes rich in P450 enzymes or cytosols rich in reductases or soluble peroxidases) from rats by simple differential centrifugation (105,000 x g).
NOTE: The pellet and supernatant are taken as microsomes and cytosols, respectively.- Wash the liver samples (1-10 g) twice with 50 mM Tris-HCl buffer, pH 7.4 containing 150 mM KCl buffer (buffer 1) (10-times higher volumes than weight of the tissue, i.e. 10-100 mL) and cut the tissues into small pieces (of around the 2 x 2 mm size).
- Homogenize the tissue in the presence of this buffer (>3 volume/weight mL/g) in homogenizer at 4 °C for 5 min, and discard the residual non-homogenized pieces of the tissue by filtration using filter paper. Centrifuge the homogenate at 600 x g for 10 min at 4 °C and transfer the supernatant to another centrifugation tube.
- Re-homogenize the pellet in buffer 1 (1 mL per 1 g of tissue), repeat step 1.1.3., and discard the pellet. Centrifuge pooled supernatants at 15,000 x g for 20 min at 4 °C. Transfer the supernatant to another centrifugation tube.
- Centrifuge the supernatant at 105,000 x g for 60 min at 4 °C. Collect the supernatant (cytosol) and store it in aliquots (1 – 10 mL) at -80 °C. Characterize cytosol for the amount of protein using the method described by Bradford26.
- Re-suspend the pellet in 100 mM sodium phosphate buffer, pH 7.4 (>2 volume/weight mL/g), centrifuge at 105,000 x g for 60 min at 4 °C. Discard the supernatant. Re-homogenize the pellet (microsomes) in 50 mM Tris-HCl buffer, pH 7.4 containing 150 mM KCl and 20% glycerol (<5 volume/weight mL/g) in homogenizer at 4 °C. Store microsomes in 0.5 – 1 mL aliquots at -80 °C. Characterize microsomes for the content of proteins using the method described by Bradford26.
- Determine the concentration of cytochrome P450 in microsomes.
NOTE: The concentration of P450 enzymes in microsomes is measured as described by Omura and Sato27, determining the absorption of the complex of reduced P450 with carbon monoxide (CO). Carbon monoxide is a toxic compound, and has to be handled with care and in a hood.
2. Incubations of Test Chemicals (Carcinogens/Drugs) with DNA in the Presence of Enzymatic Systems
- Incubation of test chemicals (carcinogens/drugs) with DNA in the presence of oxidative enzymatic systems containing cytochromes P450
- Prepare incubation mixtures containing, in a final volume of 0.75 mL at 4 °C, the following compounds and enzymatic systems.
- Mix 100 mM phosphate buffer, pH 7.4, (0.375 mL) with 10 mM NADPH or NADPH-generating system (10 mM MgCl2, 10 mM D-glucose 6-phosphate, 10 mM NADP+, 1 U/mL D-glucose 6-phosphate dehydrogenase) (75 µL).
- Add into this mixture microsomes or pure recombinant P450 in Supersomes, which are microsomes isolated from insect cells transfected with a baculovirus construct containing cDNA of recombinant P450 enzymes, 50 pmol P450 enzymes in 50 µL microsomal or supersomal preparations – hepatic microsomal fractions isolated in the laboratory or from a commercial source.
- Add 1 mg calf thymus DNA (0.3 mL of stock solution – 3.3 mg/mL in distilled water), and shake on a vortex shaker for 5 s.
- Finally, add 7.5 µL 0.1 mM test ellipticine drug dissolved in DMSO and 42.5 µL distilled water to reach a volume of incubation mixture of 0.75 mL. Use chemicals labeled with 3H or 14C or unlabelled chemicals, depending on the procedure for detection of DNA adducts.
- Shake on a vortex shaker for 5 s. Incubate in opened tubes at 37 °C for 30 – 60 min.
- Also prepare two control incubations similarly, but (i) without an activating system (microsomal samples) or (ii) with it, but without the test compound.
- Prepare incubation mixtures containing, in a final volume of 0.75 mL at 4 °C, the following compounds and enzymatic systems.
- Incubations of test chemicals (carcinogens/drugs) with DNA in the presence of reductive enzymatic systems
- Prepare incubation mixtures containing, in a final volume of 0.75 mL at 4 °C, the following compounds and enzymatic systems.
- At 4 °C, mix 100 mM Tris-HCl buffer, pH 7.4, containing 0.2% Tween 20, (0.375 mL), 10 mM solution of cofactor of NQO1 reductive enzyme (NADPH) (75 µL), cytosolic fraction (hepatic cytosolic fractions – isolated in the laboratory or in the case of using the cytosolic fractions isolated from individual human donors they were obtained from the commercial source containing 1 mg protein (50 µL)).
- Add 1 mg calf thymus DNA (0.2 mL of stock solution – 3.3 mg/ mL of distilled water), and shake on a shaker for 5 s.
- Finally, add 7.5 µL 0.1 mM test chemical (dissolved in distilled water, methanol, ethanol or DMSO, depending on solubility of the compound) and 42.5 µL distilled water to reach a volume of 0.75 mL for the incubation mixture. Use chemicals labeled with 3H or 14C or unlabelled chemicals, depending on the procedure for detection of DNA adducts (see below).
- Purge the reaction mixture with argon for 1 min. Shake on a shaker for 5 s. Incubate in closed tubes at 37 °C for 30 – 60 min.
- Also prepare two control incubations similarly, but (i) without an activating system (cytosolic fractions) or (ii) with it, but without the test chemicals.
- Prepare incubation mixtures containing, in a final volume of 0.75 mL at 4 °C, the following compounds and enzymatic systems.
- Extraction of incubation mixtures with organic solvents to remove the excess of test chemicals
- Mix the incubation mixture in a test tube with a cap with the same volume of ethyl acetate (or diethyl ether or hexane) by adding these solvents. Shake the contents of the tube on a shaker until an emulsion forms.
- Spin (3 min) at 1,600 x g in a centrifuge at ambient temperature. If the organic and aqueous phases are not properly-separated, spin once more for a longer period or at a higher centrifugation speed.
- Remove the upper, organic phase collecting with a pipette. If small volumes (<400 µL) are used, utilize an automatic pipette fitted with a suitable tip. Cast aside this organic phase.
- Repeat steps 2.3.1., 2.3.2., and 2.3.3. Remove residual organic solvents by a stream of nitrogen gas (at least 5 – 10 min of removal are needed).
- Isolation of DNA from incubations
- Extraction of DNA from solutions with phenol/chloroform and its precipitation with ethanol
- Eliminate proteins to isolate DNA from incubation mixtures by extracting proteins from solutions of DNA with phenol, phenol/chloroform (1:1), and chloroform by the procedure shown below.
- Combine the incubation mixture with the same amount of phenol or phenol/chloroform (1:1) in a Falcon or Eppendorf tube with a cap. Stir the mixture until an emulsion forms.
- Spin (3 min) at 1,600 x g in a centrifuge at ambient temperature. If the organic and aqueous phases are not properly separated, spin once more for a longer period or at a higher centrifugation speed.
- Transfer the upper water phase with a pipette to a new polypropylene tube. If small volumes (<400 µL) are used, utilize an automatic pipet fitted with a suitable tip. Remove the protein interface together with the organic phase.
- Combine the upper water phase with the same amount of a mixture of phenol and chloroform (1:1). Reproduce steps 2.4.1.2. – 2.4.1.4.
- Combine the upper water phase with the same amount of chloroform and repeat steps 2.4.1.2. – 2.4.1.4. Recover the DNA by precipitation with 2 volumes of cold (-20 °C) ethanol. Determine the volume of the DNA solution.
- Adapt the concentration of monovalent cations by addition of 5 M sodium chloride to the final concentrations of 0.1 M. Stir vigorously. Combine with 2 volumes of cold (-20 °C) ethanol and mix properly. Cool to -20°C.
- Store at -20 °C until DNA is precipitated. When DNA is fragmented during the incubations (e.g., by formation of oxygen radicals during the enzymatic activation reaction) or during the isolation procedure to the size of DNA that is small (<1 kb) or when DNA is present in small amounts (<0.1 mg/mL), the time of cooling has to be increased and the temperature decreased to -70 °C.
- Spin (10 min) at 1,600 x g at 0 °C in a centrifuge. If DNA is present at low concentrations or in the form of small fragments, spin once more for a longer period (30 min). Discard the supernatant.
- To remove any solutes (or residual traces of the test chemical) which might be present in precipitated DNA, wash DNA with 70% ethanol and diethyl ether. Wash precipitated DNA with cold (-20 °C) 70% ethanol. Spin (10 min) at 1,600 x g at 0 °C in a centrifuge. Discard the supernatant.
- Repeat the step 2.4.1.10. Wash precipitated DNA with cold (-20 °C) 70% ethanol. Spin (10 min) at 1,600 x g at 0 °C in a centrifuge. Discard the supernatant.
- Repeat the step 2.4.1.10. Put the tube into a vertical state on absorbent paper to remove the residual supernatant. Wash the DNA pellet by adding 1 mL of diethyl ether to discard the potential residues of the test chemical from isolated DNA. Spin (10 min) at 1,600 x g at 0 °C in a centrifuge. Discard the supernatant.
- Dissolve the DNA pellet in the appropriate volume (usually in 100 – 400 µL to achieve a DNA concentration of 0.5 – 1 µg/µL) of distilled water (or in 0.15 mM sodium citrate and 1.5 mM sodium chloride). The DNA solution can stand at 4 °C overnight, or can be heated to 37 °C for 10-30 min to increase dissolving DNA.
- Before storage, separate the DNA into small aliquots (10 – 20 µL), because repeated freezing and thawing of DNA solutions might result in a decrease in adduct concentrations. Store at -80 °C or colder.
NOTE: Spectrophotometric determination of DNA: The simple and accurate method, which is widely used to measure the amount of DNA in a preparation if the sample is pure (i.e., without significant amounts of contaminants such as protein, phenol, or other nucleic acids), is spectrophotometric measurement of the amount of DNA of UV irradiation absorbed by the bases (see the procedures described previously)28.
- Extraction of DNA from solutions with phenol/chloroform and its precipitation with ethanol
- Procedures for detection of DNA adduct formation
- 32P-postlabeling assay
NOTE: DNA hydrolysis utilizes the hydrolysate prepared at this stage for the analysis of adducts (2.5.1.) as well as of normal (unmodified) deoxynucleotides (2.5.7.).- Dissolve micrococcal nuclease (MN) in water at a concentration of ~450 units (U)/mL. Dialyze against distilled water and adjust to 300 U/mL. Dialyze spleen phosphodiesterase (SPD) solution and adjust to 4 U/mL.
- Mix MN and SPD to final concentrations 150 mU/µL MN and 2.5 mU/µL SPD (MN/SPD solution). Take DNA solution containing 12.5 µg, and evaporate to dryness in an evaporator. Dissolve in 6.5 µL distilled water.
- Add 5.0 µL MN/SPD solution (final concentration of MN is 60 mU/µL, final concentration of SPD is 1 mU/µL), and 1.0 µL digestion buffer (final concentration of sodium succinate is 20 mM, final concentration of CaCl2 is 8 mM). The final volume of the mixture is 12.5 µL. Mix and allow reactions for 3 h at 37 °C.
- Remove 2.5 µL (transfer to another tube) for further dilution and analysis of unmodified deoxynucleotides (2.5.7.)
- Nuclease P1 enrichment procedure
- To the remaining 10.0 µL of hydrolysate, add 0.65 µL sodium acetate buffer (final concentration, 40 mM), 0.65 µL ZnCl2 solution (final concentration 0.1 mM), 1.25 µL NP1 solution (final concentration, 0.385 µg/µL), and 0.45 µL distilled water. The final volume of the mixture is 13 µL.
- Allow the mixture to react for 30 min at 37 °C, and end the reaction with addition of 3 µL of Tris solution.
- n-Butanol enrichment procedure
- To the remaining 10.0 µL of DNA hydrolysate, add 215 µL of 11.6 mM ammonium formate solution, pH 3.5, and 25 µL 10 mM TBA chloride solution. Extract with 250 µL n-butanol (saturated with water) by vigorous mixing. Spin (3 min) at 1,600 x g to separate layers, and take off the upper n-butanol layer. Extract once more with 250 µL n-butanol (saturated with water), spin, take off the upper n-butanol layer, and combine with former extract.
- Add 400 µL water (saturated with n-butanol) to this extract, and mix vigorously. Spin to separate layers and delete the bottom aqueous layer. Repeat this washing procedure using 400 µL of water (saturated with n-butanol). Add 3 µL of 250 mM Tris-HCl solution, pH 9.5, to the n-butanol layer. Evaporate n-butanol to dryness in an evaporator at ambient temperature.
- Dissolve the residue in 100 µL n-butanol, evaporate to dryness again, and dissolve the residue in 16.0 µL water.
- Labeling of the adducts
- Add 1 µL of bicine buffer solution (labeling buffer) and 4.5 µL of a mix containing 100 µCi [γ-32P]ATP, 45 pmol of cold ATP, and 10.0 U of T4-PNK (T4-phosphonucleotide kinase) to 16.0 µL solution from NP1 or butanol enrichment mix. The final concentrations of the reagents will be the following: 20 mM bicine, 10 mM MgCl2, 10 mM dithiotreitol, 0.5 mM spermidine, and 0.5 U/µL T4-PNK, 3 µM ATP. The total volume of the mixture is 20 µL.
- Allow the mixture to react for 30 min at room temperature. Apply the whole sample (i.e. 20 µL) onto the PEI-cellulose TLC plate (2.5.6.).
- Evaluation of efficacy of NP1 or n -butanol enhancing procedures
- Wash the bottom of the tube with 50 µL water. Mix vigorously for 30 s and spin (1 min) at 1,600 x g in a centrifuge to establish there is no contamination on the lid. Spot 5 µL on a PEI-cellulose TLC plate (20 x 20 cm). Chromatograph using a solution 280 mM in (NH4)2SO4 and 50 mM NaH2PO4, pH 6.8.
- TLC separation of adducted deoxynucleotides
- Pre-wash the TLC plates with distilled water. It is recommended especially for home-made plates.
NOTE: This wash should be carried out to remove the yellow color from plates, a color which can elevate the background, particularly at the solvent front. - Spot the whole sample onto the PEI-cellulose TLC plate and start chromatography (2.5.4.); clean-up of adducts is performed by developing the plate in the D1 and D2 directions (Figure 2).
- Develop the plate in a D1 direction (Figure 2). Use 1.7 M sodium phosphate buffer, pH 6.8, to ensure that the DNA adducts are remaining at the start of the TLC plate. Analogously, develop the plate in a D2 direction using this buffer. For information on possible buffers for procedures, see solutions described for resolution of several types of adducts20,28.
NOTE: Development of the plate in a D2 direction can be omitted. - Wash the plate in deionized water after chromatography for about 5 min in two consecutive baths. After that, dry the plates.
- Develop the plates in D3 and D4 direction using 3.5 M lithium formate buffer, pH 3.5, containing 8.5 M urea for D3 direction and 0.5 M Tris-HCl buffer, pH 8.0, containing 0.8 M LiCl and 8.5 M urea for D4 direction (Figure 2). The solvents must be adjusted to develop the adduct spots over the TLC plate. For information on possible buffers for D3 and D4 developing procedures, see the solutions described for resolution of several types of adducts23,24,25,28.
- To avoid any problems in D4, after development in a D4 direction and a water wash, develop the plates (along D4) in 1.7 M sodium phosphate, pH 6.0 (usually assigned as development in direction D5), to the top of a paper wick (12 x 11.5 cm).
NOTE: The D5 direction can also be omitted. In this case, it is necessary to open the TLC tank when the solvent has reached the top of the TLC, and allow a run for up to 60 min. This method is even better than adding a wick (the method frequently used in many laboratories to delete any problems in D4).
- Pre-wash the TLC plates with distilled water. It is recommended especially for home-made plates.
- Quantification of normal (unmodified) deoxynucleotides after hydrolysis
- Dilute an aliquot of hydrolysate (from 2.5.1. describing DNA hydrolysis) with distilled water, (i.e., 2.5 µL of the digestion mixture from 2.5.1. adjusted to 250 µL, and 10 µL of this solution adjusted to 150 µL).
- Take a 5 µL (10 pmol normal deoxynucleotides) aliquot of this digest, add 2.5 µL 10 mM Tris-HCl buffer, pH 9.0, and label as in 2.5.4. (the final volume of the mixture is 10 µL). Allow to react for 30 min at room temperature.
- Take a 4 µL aliquot of the mixture and dilute to 750 µL with 10 mM Tris-HCl, pH 9.0. Mix and spin to establish there is no contamination of the lid. Apply 5 µL on a PEI-cellulose TLC plate. Develop the TLC plate in a solution of 280 mM (NH4)2SO4 and 50 mM NaH2PO4, pH 6.5. Allow to dry after TLC.
- Use autoradiography that is performed for approximately 45 min at ambient temperature to localize the four unmodified deoxynucleotide bis-phosphates. Cut spot for quantification by either liquid scintillation or Cerenkov counting.
- Calculation of the relative adduct labeling (RAL)
- Determine the values of the counts in the adduct spots and the counts in the aliquot of labeled unmodified (normal) deoxynucleotides.
NOTE: The latter have to be determined on 180,000 counts less material than the former, a figure which is the conversion factor to apply to the count of unmodified (normal) deoxynucleotides for evaluating the RAL values of adduct levels. RAL of DNA adducts is calculated according to the equation (2) shown above.
- Determine the values of the counts in the adduct spots and the counts in the aliquot of labeled unmodified (normal) deoxynucleotides.
- Detection of binding of the test carcinogens or drugs to DNA using radioactive-labeled drug
- Evaluate the 3H or 14C radioactivity of DNA modified with chemicals by liquid scintillation counting.
- Add 10 – 50 µL of DNA solution to 3 mL of a scintillation solution in the scintillation vial. Mix well. Measure the radioactivity using the scintillation counter.
- 32P-postlabeling assay
Representative Results
Using the protocols described here for the utilization of enzyme-catalyzed activation (i.e., P450, peroxidase, reductase) to investigate potency of chemicals (carcinogens/drugs) to be metabolized to intermediates resulting in their covalent binding to DNA (generation of DNA adducts), we were able to resolve (i) a novel mechanism of the pharmacological action of the anticancer agent ellipticine (for a review see,29,30,31), (ii) the etiology of two nephropathies associated with upper urothelial tract cancer caused by plant alkaloid aristolochic acid (Aristolochic acid nephropathy and Balkan endemic nephropathy) (for a review see,32,33,34,35,36,37,38,39), and (iii) the genotoxic mechanisms of carcinogenicity of several carcinogens such as an air pollutant 3-nitrobenzanthrone (3-NBA)40,41,42,43,44,45 and its reductive counterpart, 3-aminobenzanthrone (3-ABA),46,47,48 plant alkaloid aristolochic acid,32,33,34,35,36,37,38,39 and an aromatic amine o-anisidine.49,50,51,52 Further, the enzymes determining biological effects of these chemicals were determined employing the described methods.
Here, representative results on oxidative activation of ellipticine by P450s and peroxidases resulting in generation of covalent adducts with DNA, and on reduction of 3-NBA to metabolites that covalently modified DNA, are shown.
The plant alkaloid ellipticine (5,11-dimethyl-6H-pyrido[4,3-b]carbazole) and its derivatives are antitumor agents, which act as DNA-damaging drugs through several mechanisms, included into arrest of cell cycle and induction of apoptosis (for an overview, see29,30,31). Using the protocols described in this work, we demonstrated that the anticancer drug generates covalent DNA adducts after metabolic bioactivation catalyzed by microsomal P450s (Figure 3) and peroxidases (Figure 4)29,30,31,53,54,55,56,57,58,59,60,61, and this explained the strong efficiency of this agent against cancer cells30. The [3H]-radiolabeled ellipticine and the nuclease P1 version of the 32P-postlabeling technique were predominantly utilized in the studies29,30,31,53,61. Using the complex study with subcellular enzyme systems, the enzyme inhibitors and pure enzymes in the experiments utilizing the described protocols, the predominant P450 enzymes oxidizing ellipticine to reactive species forming DNA adducts and structures of these reactive species were characterized29,30,31,55. Of the P450s examined, the human CYP3A4 enzyme is most efficient in ellipticine oxidation to 12-hydroxyellipticine and 13-hydroxyellipticine, the ellipticine metabolites, which spontaneously decompose to ellipticine-12-ylium and ellipticine-13-ylium binding to DNA (Figure 5).55,61 The CYP enzymes also generate further metabolites such as 9-hydroxyellipticine, which is considered a detoxification metabolite, 7-hydroxyellipticine and ellipticine N2-oxide, which are formed as the minor ellipticine metabolites. The 9-hydroxyellipticine as well as 7-hydroxyellipticine and ellipticine N2-oxide are mainly formed by CYP1A1 and CYP2D6, respectively.55,57,58
Peroxidases (i.e., horseradish peroxidase (HRP), lactoperoxidase (LPO), myeloperoxidase (MPO) and cyclooxygenases (COX-1 and COX-2)) metabolize ellipticine to generate the same ellipticine-derived DNA adducts (Figure 4)61 by the mechanisms shown in Figure 5.
The nitroaromatic 3-NBA (3-nitro-7H-benzdeanthracen-7-one) is a component of diesel exhaust and is found in airborne particles62,63,64. The major metabolite of this pollutant, 3-ABA,64,65 was detected in urine of workers of salt mines that were exposed to diesel emissions for a long time63. This finding demonstrated that these workers were exposed to 3-NBA. This nitroaromatic causes lung tumors in rats after intratracheal instillation67. 3-NBA also acts as a mutagen in the Ames Salmonella typhimurium test (in strain YG1024 overexpressing nitroreductase and O-acetyltransferase), generating more than 6 million revertants per nanomole in this strain62. Its genotoxic potency was also demonstrated by generation of covalent adducts with DNA in vitro, after activation by several enzymes, using the protocols described in this work, and in vivo in several organs of rodent animals (Figure 6)39,40,41,42,43,44,45,46,47,64,67,68.
The 3-NBA-derived DNA adducts formed after 3-NBA activation with cytosolic reductases (i.e., NQO1) were measured by the nuclease P1 and n-butanol enrichment methods of the 32P-postlabeling method described in the protocols presented in this study. The results indicated the formation of up to five DNA adducts (adduct spots 1-5 in Figure 7), and three of them were characterized to be 2-(2'-deoxyadenosin-N6-yl)-3-aminobenzanthrone (dA-N6-C2-ABA; adduct spot 1), N-(2'-deoxyguanosin-N2-yl)-3-aminobenzanthrone (dG-N2-C2-ABA; adduct spot 3) and N-(2'-deoxyguanosin-8-yl)-3-aminobenzanthrone (dG-C8-N-ABA; adduct spots 4 and 5) (Figure 7 and Figure 8). Utilizing the nuclease P1 version of the 32P-postlabeling method, the dG-C8-N-ABA (adduct spots 4 and 5) was undetectable (Figure 7 and Figure 8). This result underlines that some limitations of this version of 32P-postlabeling occur, namely, the low (if any) detection of adducted deoxynucleotides that are dephosphorylated by nuclease P1 (i.e., adducts formed during oxidation of arylamines or by reduction of aromatic nitroderivatives to N-hydroxyarylamine-derivatives substituted at C8 of deoxyguanosine). Similar to the study with ellipticine, utilizing the complex study with subcellular enzyme systems, the enzyme inhibitors, pure enzymes, and DNA adduct standards in the experiments employing the protocols described in this work, the predominant cytosolic reduction enzymes metabolizing 3-NBA to metabolites generating DNA adducts, namely, the reactive metabolite formed by reduction of 3-NBA (N-OH-ABA), and structures of three DNA adducts generated by 3-NBA were characterized (Figure 7 and Figure 8). In the liver, bioactivation of 3-NBA in vitro was found to be mainly attributable to human and rat NQO1 (Figure 7), while human N,O-acetyltransferases (NATs), NAT2, NAT1, sulfotransferase (SULT), SULT1A1 and, to a lesser extent, SULT1A2 are the predominant enzymes of phase II activating 3-NBA42. Hepatic microsomal POR is also effective in the activation of 3-NBA41, but in mice, 3-NBA is mainly bioactivated by NQO1 rather than this microsomal POR42. In lung, which is the target tissue for 3-NBA carcinogenicity67, both NQO1 and XO reduce 3-NBA to metabolites generating DNA adducts. However, XO seems to act as a minor 3-NBA activating enzyme in this organ69.
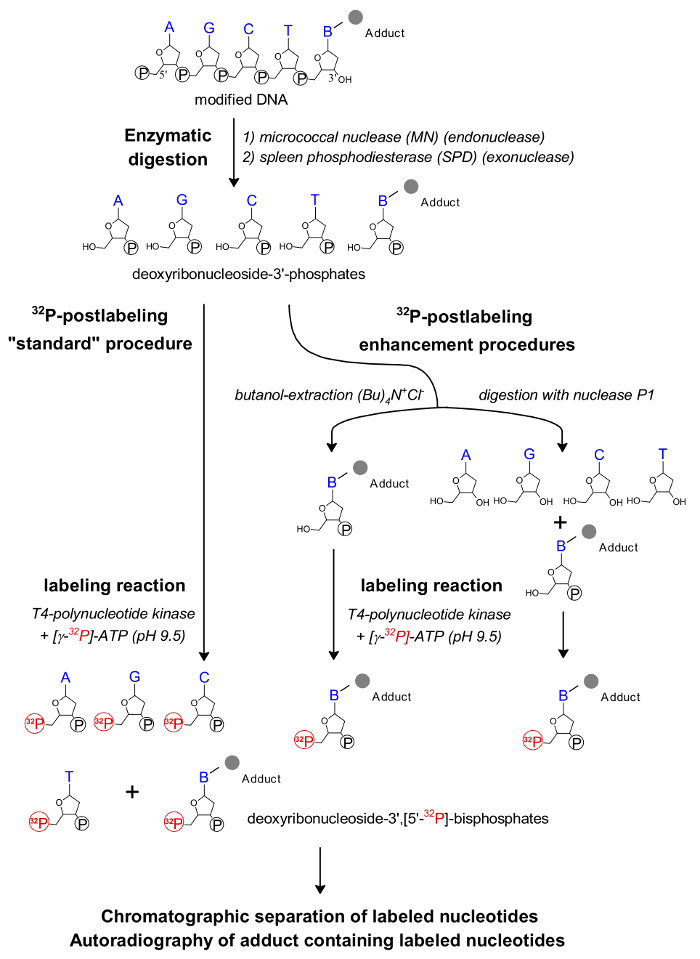
Figure 1: Scheme of the 32P-postlabeling assay. The individual steps of the 32P-postlabeling method and its enhanced procedures are shown. Please click here to view a larger version of this figure.
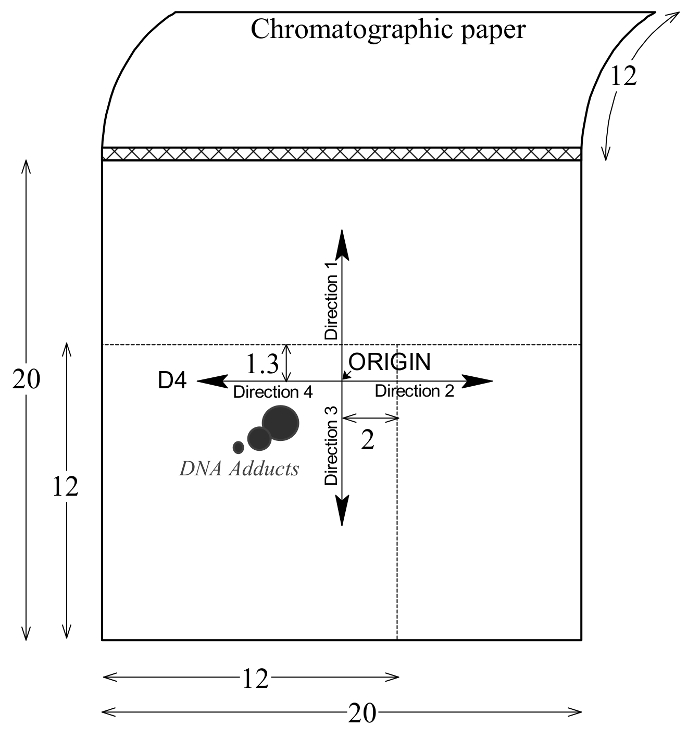
Figure 2: Pattern of DNA adduct elution on PEI-cellulose TLC plates. The multidirectional chromatography of DNA adducts on PEI-cellulose plates are shown. ORIGIN is a start position on the PEI-cellulose TLC plate. Please click here to view a larger version of this figure.
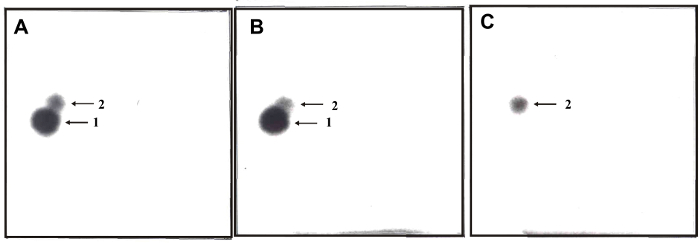
Figure 3: 32P-postlabeling analyses of DNA adducts formed in calf thymus DNA incubated with ellipticine, NADPH and (A) rat and (B) human hepatic microsomes, (C) a control sample without microsomes. Adducts 1 and 2 assigned by arrows are generated in deoxyguanosine in DNA by ellipticine activated with microsomes.32P-postlabeling was performed employing the nuclease P1 version of the method (Step 2.5.2.) Origins are located at the bottom left corners (D3 from bottom to top and D4 from left to right). D2 was omitted. Please click here to view a larger version of this figure.
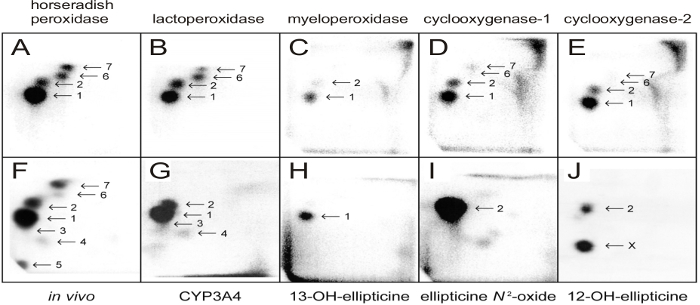
Figure 4: 32P-postlabeling analyses of ellipticine-mediated DNA adducts. Adducts formed in calf thymus DNA reacted with ellipticine (100 µM) and horseradish peroxidase (A), bovine lactoperoxidase (B), human myeloperoxidase (C), ovine cyclooxygenase-1 (D), human cyclooxygenase-2 (E) (5 µg peroxidases were present in the incubations), from liver DNA of rats treated with 40 mg ellipticine per kilogram body weight (bw) (F), from calf thymus DNA reacted with ellipticine and human CYP3A4 (G), with 13-hydroxyellipticine(H), ellipticine N2-oxide (I), and 12-hydroxyelipticine (J). Experiments were performed using the nuclease P1 version of the assay (step 2.5.2.) Origins are at the bottom left corners (D3 from bottom to top and D4 from left to right). D2 was omitted. Please click here to view a larger version of this figure.
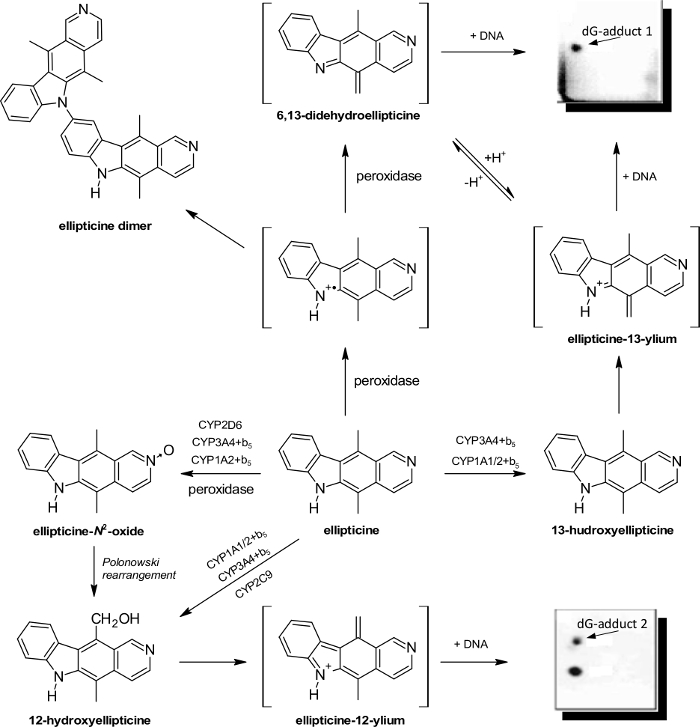
Figure 5: Oxidation of ellipticine by peroxidases and CYPs showing the ellipticine metabolites and those suggested to generate DNA adducts. The compounds in brackets have not still been detected under the experimental conditions used in the experiments, and they are the electrophilic metabolites postulated as ultimate species binding to DNA. Please click here to view a larger version of this figure.
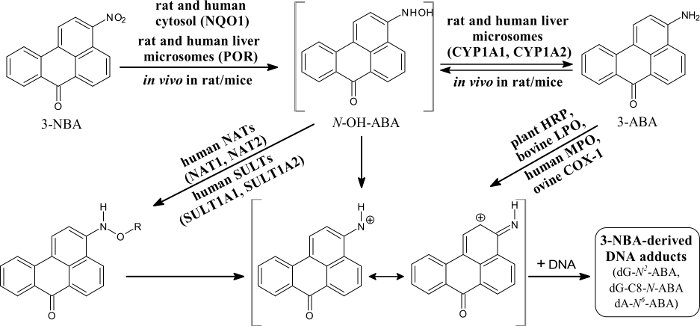
Figure 6: Metabolic activation of and DNA adduct formation by 3-nitrobenzanthrone. 3-NBA, 3-nitrobenzanthrone; NQO1, NAD(P)H:quinone oxidoreductase; NAT, N,O-acetyltransferases; SULT, sulfotransferase; CYP, cytochrome P450; POR, NADPH:cytochrome P450 oxidoreductase; HRP, horseradish peroxidase; LPO, lactoperoxidase; MPO, myeloperoxidase; COX-1, cyclooxygenase-1. R = -COCH3 or -SO3H; dA-N6-ABA, 2-(2'-deoxyadenosin-N6-yl)-3-aminobenzanthrone; dG-N2-ABA, N-(2'-deoxyguanosin-N2-yl)-3-aminobenzanthrone; dG-C8-N-ABA, N-(2'-deoxyguanosin-8-yl)-3-aminobenzanthrone. Please click here to view a larger version of this figure.
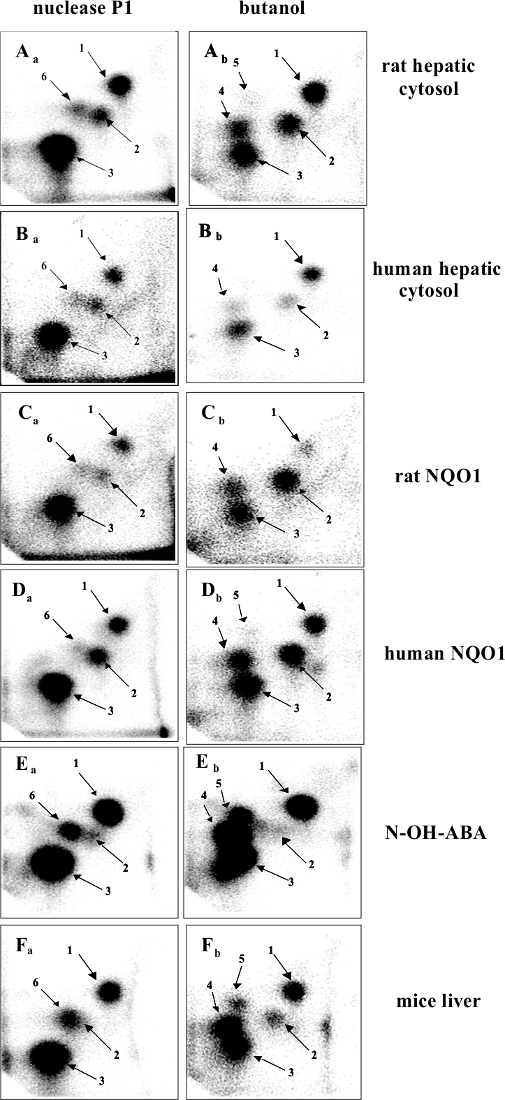
Figure 7: 32P-postlabeling analyses of 3-NBA-derived DNA adducts. The nuclease P1- (left panels) and n-butanol extraction versions (right panels) of the method were utilized. Aa and Ab, adducts formed in calf thymus DNA reacted with 3-NBA (300 µM) after activation with rat hepatic cytosols. Ba and Bb, adducts formed in calf thymus DNA, reacted with 3-NBA (300 µM) after activation with human hepatic cytosol (pooled fraction). Ca and Cb, adducts formed in calf thymus DNA, reacted with 3-NBA (300 µM) after activation with pure rat hepatic NQO1 (0.09 units). Da and Db, adducts formed in calf thymus DNA, reacted with 3-NBA (30 µM) after activation with human recombinant NQO1 (0.06 units). Ea and Eb, adducts formed in salmon testis DNA treated with N-OH-ABA. Fa and Fb, adducts formed in liver DNA of wild-type littermates on a C57BL/6 background exposed to 2 mg of 3-NBA per kg b.w. Please click here to view a larger version of this figure.
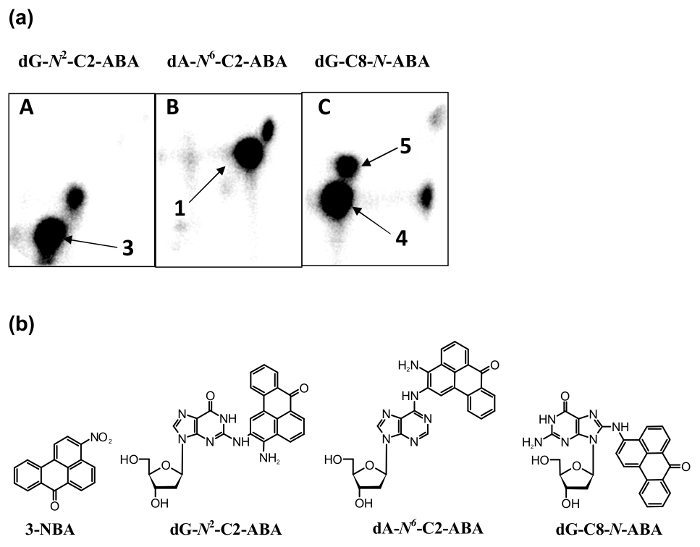
Figure 8: 32P-postlabeling analyses of 3-NBA-derived DNA adduct standards [dG-N2-C2-ABA (A), dA-N6-C2-ABA (B) and dG-C8-N-ABA (C)] (panels a) and structures of 3-NBA and these 3-NBA-DNA adducts (panels b). The n-butanol extraction version of the method was utilized (panels in a). Please click here to view a larger version of this figure.
Discussion
In this paper, it is demonstrated a widely accessible methodology to study the potency of chemicals to be bioactivated to metabolic intermediates, resulting in generation of covalent DNA adducts. This is a crucial issue, because evaluation of the potency of environmental chemicals or drugs of their enzymatic activation to metabolites generating covalent DNA adducts is an important field in the development of cancer and its treatment. The modification of DNA by carcinogens considered the cause of tumor development is now judged as a central dogma of carcinogenesis caused by carcinogens. This suggestion is confirmed by a variety of findings, such as the phenomena that: (i) the carcinogenic properties of various carcinogens depend on bioactivation of these compounds to metabolites reacting with nucleophilic centers in DNA; (ii) levels of DNA adducts frequently correspond to many carcinogenic responses; (iii) and the mutation in certain tumor suppressor genes and the activation of several proto-oncogenes might be caused by their modification with chemicals with carcinogenic potencies. Moreover, the covalent modification of DNA by anticancer drugs has been demonstrated as one of the most efficient DNA-damaging effects of these drugs, which results in their use in cancer treatment.
There are two critical points which determine the successful evaluation of chemicals for their genotoxic properties, i.e. their potencies to form covalent DNA adducts, specifically: (i) to find, resolve, and characterize the efficiency of enzymatic systems capable of activating carcinogens/drugs to electrophilic species binding the nucleophilic centers of DNA, and (ii) to develop and use the most suitable techniques, by which carcinogen/drug–DNA adducts are found and structurally characterized. The appropriate methods for both features are described in this study.
Isolation procedures for cellular fractions containing biotransformation enzymes (microsomal or cytosolic samples possessing P450s and additional bioactivating enzymes i.e. peroxidases or reductases POR, NQO1, XO and AO), the protocols for bioactivation of test carcinogens/drugs by the enzymatic systems (incubations with DNA), and their use shown in this work indicated, as demonstrated by the representative results, their suitability for evaluation of genotoxic properties of chemicals.
Further, the methods appropriate for detection and quantification of adducts with DNA such as two enrichment versions of the 32P-postlabeling technique (the nuclease P1- and n-butanol extraction procedures) and the use of radioactively-labeled tested compounds were shown to be appropriate for studies evaluating genotoxicity of carcinogen/drug xenobiotics.
Concerning the determination of the covalent DNA adducts, not only these two methods, but also other methods suitable for detection and measurement of DNA adducts have been established8,70,71,72,73,74,75,76,77,78,79,80,81,82. Until 1981, the quantification of DNA adducts utilized radioactive chemicals (carcinogens/drugs), labeled by 3H or 14C, that have been prepared synthetically. Such methods have been utilized usefully for studies with ellipticine as described in this work (see53,54). Nevertheless, it is usually difficult to prepare the derivatives with high radioactivity for their successful usage73,80,81. Therefore, even though this procedure is still used, it is unfortunately limited to in vitro experiments similar to those described here. Of other techniques, mass spectrometry, electron spray ionization (ESI), matrix-assisted laser desorption ionization (MALDI), accelerator mass spectrometry (AMS), fluorescence, biological methods such as immunoassay, and 32P-postlabeling described in this study in detail, were developed (for review, see8,16,70,71,72,73,74,75,76,77,78,79,80,81,82).
The 32P-postlabeling assay described in the protocol of this work is shown, which not only has applicability in the in vitro experiments (see Representative Results), namely, testing new compounds for genotoxicity or mechanistic investigations of carcinogen/drug activation, but has also further utilizations such as evaluating human exposure to environmental carcinogens, studies on the mechanisms of tumor development, monitoring repair of DNA, examining DNA damage by endogenous compounds and oxidative reactions, and investigating the response of patients to cytotoxic anticancer drugs72,83.
This technique is, however, not without some limitations73. The lesions in DNA, which are not stable as monodeoxynucleotides, cannot be determined reliably. The 32P-postlabeling method is not capable of identifying the structures of DNA adduct. Therefore, structural characterization of DNA adducts frequently relies on demonstrating their co-chromatography with synthetic standards of known structures. Indeed, such a method was used in both examples of the representative results shown in this work (ellipticine, 3-NBA).
In conclusion, the protocols shown in this work may be considered as suitable methods to evaluate the potency of environmental chemicals or drugs to be enzymatically bioactivated to metabolites generating covalent DNA adducts, a highly important process for the development of cancer and its treatment.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This study was supported by Czech Science Foundation (GACR, grant 17-12816S).
Materials
| Tris | Sigma-Aldrich | 252859 | |
| Potassium chloride | Sigma-Aldrich | P9541 | |
| Sodium acetate | Sigma-Aldrich | S2889 | |
| Phenol | Roth | 0032.8 | |
| Phenol/Chloroform/Isoamylalcohol | Roth | A156.1 | |
| Ethanol | Penta | 70390-11000 | |
| Calf thymus DNA | Sigma-Aldrich | D4522 | |
| NADH | Sigma-Aldrich | N7004 | |
| NADP+ | Sigma-Aldrich | N5755 | |
| NADPH | Sigma-Aldrich | N7505 | |
| D-glucose 6-phosphate | Sigma-Aldrich | 7647001 | |
| D-glucose 6-phosphate dehydrogenase | Sigma-Aldrich | G6378 | |
| Supersomes | Corning Gentest | 456211, 456203, 456220, 456204, 456210, 456222, 456219, 456212, 456206, 456207, 456202 | |
| Human liver microsomes | Corning Gentest | 452172 | |
| Tween 20 | Sigma-Aldrich | P1379 | |
| Hypoxanthine | Sigma-Aldrich | 77662 | |
| 2-Hydroxypyrimidine | Sigma-Aldrich | H56800 | |
| Ethyl acetate | Sigma-Aldrich | 437549 | |
| Diethyl ether | Sigma-Aldrich | 179272 | |
| Micrococcal nuclease from Staphylococcus aureus | Sigma-Aldrich | N3755 | |
| Spleen phosphodiesterase from calf spleen, Type II | Calbiochem | 524711 | |
| Nuclease P1 from Penicillium citrinum | Sigma-Aldrich | N8630 | |
| Bicine | Sigma-Aldrich | 163791 | |
| DL-Dithiotreitol | Sigma-Aldrich | D0632 | |
| Spermidine | Sigma-Aldrich | S2626 | |
| Tetrabutylammonium chloride | Sigma-Aldrich | 86870 | |
| n-Butanol | Sigma-Aldrich | 437603 | |
| T4-polynucleotide kinase | USB Corp | 70031Y | |
| [γ-32P]ATP | Hartman Analytic GmbH | FP-201 | |
| PEI-impregnated cellulose TLC plates | Macherey-Nagel | 801053 | |
| Packard Instant Imager A202400 | Packard | G120337 | |
| Ellipticine | Sigma-Aldrich | 285730 | |
| 3-Nitrobenzanthrone | prepared (synthesized) as shown in ref. 40 | ||
Referanslar
- Croom, E. Metabolism of xenobiotics of human environments. Prog. Mol. Biol. Transl. Sci. 112, 31-88 (2012).
- Guengerich, F. P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 14 (6), 611-650 (2001).
- Guengerich, F. P. Cytochrome P450 and chemical toxicology. Chem. Res. Toxicol. 21 (2), 70-83 (2008).
- Stiborova, M., et al. NADH:Cytochrome b5 Reductase and Cytochrome b5 Can Act as Sole Electron Donors to Human Cytochrome P450 1A1-Mediated Oxidation and DNA Adduct Formation by Benzo[a]pyrene. Chem. Res. Toxicol. 29 (8), 1325-1334 (2016).
- Rendic, S., Guengerich, F. P. Contributions of human enzymes in carcinogen metabolism. Chem. Res. Toxicol. 25 (7), 1316-1383 (2012).
- Wienkers, L. C., Heath, T. G. Predicting in vivo drug interactions from in vitro drug discovery data. Nat. Rev. Drug Discov. 4 (10), 825-833 (2005).
- Guengerich, F. P., Liebler, D. C. Enzymatic activation of chemicals to toxic metabolites. Crit. Rev. Toxicol. 14 (3), 259-307 (1985).
- Poirier, M. C. Linking DNA adduct formation and human cancer risk in chemical carcinogenesis. Environ. Mol. Mutagen. 57 (7), 499-507 (2016).
- Rappaport, S. M., Li, H., Grigoryan, H., Funk, W. E., Williams, E. R. Adductomics: characterizing exposures to reactive electrophiles. Toxicol. Lett. 213 (1), 83-90 (2012).
- Luch, A. Nature and nurture – lessons from chemical carcinogenesis. Nat. Rev. Cancer. 5 (2), 113-125 (2005).
- Nebert, D. W., Dalton, T. P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 6 (12), 947-960 (2006).
- Phillips, D. H. . Macromolecular adducts as biomarkers of human exposure to polycyclic aromatic hydrocarbons. , 137-169 (2005).
- Phillips, D. H. DNA adducts as markers of exposure and risk. Mutat. Res. 577 (1-2), 284-292 (2005).
- Hemminki, K. DNA adducts, mutations and cancer. Carcinogenesis. 14 (10), 2007-2012 (1993).
- Hanawalt, P. C., Spivak, G. Transcription-coupled DNA repair: two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 9 (12), 958-970 (2008).
- Geacintov, N. E., Broydem, S. Repair-resistant DNA lesions. Chem. Res. Toxicol. 30 (8), 1517-1548 (2017).
- Randerath, K., Reddy, M. V., Gupta, R. C. 32P-labeling test for DNA damage. Proc. Natl. Acad. Sci. USA. 78 (10), 6128-6129 (1981).
- Reichert, W. L., Stein, J. E., French, B., Goodwin, P., Vanarasi, U. Storage phosphor imaging technique for detection and quantitation of DNA adducts measured by the 32P-postlabeling assay. Carcinogensis. 13 (8), 1475-1479 (1992).
- Chang, L. W., Hsia, S. M. T., Chang, P. C., Hsieh, L. L. Macromolecular adducts – biomarkers for toxicity and carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 34, 41-67 (1994).
- Gupta, R. C., Reddy, M. V., Randerath, K. 32P-post-labeling analysis of nonradioactive aromatic carcinogen DNA adducts. Carcinogenesis. 3 (9), 1081-1092 (1982).
- Reddy, M. V., Randerath, K. Nuclease-P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 7 (9), 1543-1551 (1986).
- Mourato, L. L., Beland, F. A., Marques, M. M. 32P-Postlabeling of N-(deoxyguanosin-8-yl)arylamine adducts: a comparative study of labeling efficiencies. Chem. Res. Toxicol. 12 (7), 661-669 (1999).
- Randerath, E., Agrawal, H. P., Weaver, J. A., Bordelon, C. B., Randerath, K. 32P-Postlabeling analysis of DNA adducts persisting for up to 42 weeks in the skin, epidermis and dermis of mice treated topically with 7,2-dimethylbez[a]anthracene. Carcinogenesis. 6 (8), 1117-1126 (1985).
- Everson, R. B., Randerath, E., Santella, R. M., Cefalo, R. C., Avits, T. A., Randerath, K. Detection of smoking-related covalent DNA adducts in human placenta. Science. 231 (4733), 57-65 (1986).
- Gupta, R. C. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen-DNA adducts. Cancer Res. 45 (11 Pt 2), 5656-5662 (1985).
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254 (1976).
- Omura, T., Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 239, 2370-2378 (1964).
- Stiborová, M., Asfaw, B., Frei, E., Schmeiser, H. H., Wiessler, M. Benzenediazonium ion derived from Sudan I forms an 8-(phenylazo)guanine adduct in DNA. Chem. Res. Toxicol. 8 (4), 489-498 (1995).
- Stiborova, M., Rupertova, M., Schmeiser, H. H., Frei, E. Molecular mechanisms of antineoplastic action of an anticancer drug ellipticine. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 150 (1), 13-23 (2006).
- Stiborová, M., Rupertová, M., Frei, E. Cytochrome P450- and peroxidase-mediated oxidation of anticancer alkaloid ellipticine dictates its anti-tumor efficiency. Biochim. Biophys. Acta. 1814 (1), 175-185 (2011).
- Stiborova, M., Frei, E. Ellipticines as DNA-targeted chemotherapeutics. Current Med. Chem. 21 (5), 575-591 (2014).
- Arlt, V. M., Stiborova, M., Schmeiser, H. H. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis. 17 (4), 265-277 (2002).
- Arlt, V. M., et al. Aristolochic acid mutagenesis: molecular clues to the aetiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogenesis. 28 (11), 2253-2261 (2007).
- Stiborová, M., Frei, E., Arlt, V. M., Schmeiser, H. H. Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy. Mutat. Res. 658 (1-2), 55-67 (2008).
- Stiborová, M., Frei, E., Schmeiser, H. H. Biotransformation enzymes in development of renal injury and urothelial cancer caused by aristolochic acid. Kidney Int. 73 (11), 1209-1211 (2008).
- Schmeiser, H. H., Stiborová, M., Arlt, V. M. Chemical and molecular basis of the carcinogenicity of Aristolochia plants. Curr. Opin. Drug Discov. Devel. 12 (1), 141-148 (2009).
- Gökmen, M. R., et al. The epidemiology, diagnosis, and management of aristolochic acid nephropathy: a narrative review. Ann. Intern. Med. 158 (6), 469-477 (2013).
- Stiborová, M., Martínek, V., Frei, E., Arlt, V. M., Schmeiser, H. H. Enzymes metabolizing aristolochic acid and their contribution to the development of aristolochic acid nephropathy and urothelial cancer. Curr. Drug Metab. 14 (6), 695-705 (2013).
- Stiborová, M., Arlt, V. M., Schmeiser, H. H. Balkan endemic nephropathy: an update on its aetiology. Arch. Toxicol. 90 (11), 2595-2615 (2016).
- Arlt, V. M., et al. Metabolic activation of the environmental contaminant 3-nitrobenzanthrone by human acetyltransferases and sulfotransferase. Carcinogenesis. 23 (11), 1937-1945 (2002).
- Arlt, V. M., Stiborova, M., Hewer, A., Schmeiser, H. H., Phillips, D. H. Human enzymes involved in the metabolic activation of the environmental contaminant 3-nitrobenzanthrone: evidence for reductive activation by human NADPH:cytochrome P450 reductase. Cancer Res. 63 (11), 2752-2761 (2003).
- Arlt, V. M., et al. Environmental pollutant and potent mutagen 3-nitrobenzanthrone forms DNA adducts after reduction by NAD(P)H:quinone oxidoreductase and conjugation by acetyltransferases and sulfotransferases in human hepatic cytosols. Cancer Res. 65 (7), 2644-2652 (2005).
- Osborne, M. R., et al. Synthesis, characterization, and 32p-postlabeling analysis of DNA adducts derived from the environmental contaminant 3-nitrobenzanthrone. Chem. Res. Toxicol. 18 (6), 1056-1070 (2005).
- Arlt, V. M., et al. Identification of three major DNA adducts formed by the carcinogenic air pollutant 3-nitrobenzanthrone in rat lung at the C8 and N2 position of guanine and at the N6 position of adenine. Int. J. Cancer. 118 (9), 2139-2146 (2006).
- Stiborová, M., et al. Mechanisms of the different DNA adduct forming potentials of the urban air pollutants 2-nitrobenzanthrone and carcinogenic 3-nitrobenzanthrone. Chem. Res. Toxicol. 23 (7), 1192-1201 (2010).
- Arlt, V. M., Hewer, A., Sorg, B. L., Schmeiser, H. H., Phillips, D. H., Stiborova, M. 3-aminobenzanthrone, a human metabolite of the environmental pollutant 3-nitrobenzanthrone, forms DNA adducts after metabolic activation by human and rat liver microsomes: evidence for activation by cytochrome P450 1A1 and P450 1A2. Chem. Res. Toxicol. 17 (8), 1092-1101 (2004).
- Arlt, V. M., Henderson, C. J., Wolf, C. R., Schmeiser, H. H., Phillips, D. H., Stiborova, M. Bioactivation of 3-aminobenzanthrone, a human metabolite of the environmental pollutant 3-nitrobenzanthrone: evidence for DNA adduct formation mediated by cytochrome P450 enzymes and peroxidase. Cancer Lett. 234 (2), 220-231 (2006).
- Stiborová, M., et al. 3-aminobenzanthrone, a human metabolite of the carcinogenic environmental pollutant 3-nitrobenzanthrone, induces biotransformation enzymes in rat kidney and lung. Mutat. Res. 676 (1-2), 93-101 (2009).
- Stiborová, M., Miksanová, M., Havlícek, V., Schmeiser, H. H., Frei, E. Mechanism of peroxidase-mediated oxidation of carcinogenic o-anisidine and its binding to DNA. Mutat. Res. 500 (1-2), 49-66 (2002).
- Stiborová, M., Miksanová, M., Sulc, M., Rýdlová, H., Schmeiser, H. H., Frei, E. Identification of a genotoxic mechanism for the carcinogenicity of the environmental pollutant and suspected human carcinogen o-anisidine. Int. J. Cancer. 116 (5), 667-678 (2005).
- Naiman, K., Martínková, M., Schmeiser, H. H., Frei, E., Stiborová, M. Human cytochrome-P450 enzymes metabolize N-(2-methoxyphenyl)hydroxylamine, a metabolite of the carcinogens o-anisidine and o-nitroanisole, thereby dictating its genotoxicity. Mutat. Res. 726 (2), 160-168 (2011).
- Naiman, K., et al. Formation, persistence, and identification of DNA adducts formed by the carcinogenic environmental pollutant o-anisidine in rats. Toxicol. Sci. 127 (2), 348-359 (2012).
- Stiborová, M., Bieler, C. A., Wiessler, M., Frei, E. The anticancer agent ellipticine on activation by cytochrome P450 forms covalent DNA adducts. Biochem. Pharmacol. 62 (12), 1675-1684 (2001).
- Stiborová, M., Stiborová-Rupertová, M., Borek-Dohalská, L., Wiessler, M., Frei, E. Rat microsomes activating the anticancer drug ellipticine to species covalently binding to deoxyguanosine in DNA are a suitable model mimicking ellipticine bioactivation in humans. Chem. Res. Toxicol. 16 (1), 38-47 (2003).
- Stiborová, M., et al. The anticancer drug ellipticine forms covalent DNA adducts, mediated by human cytochromes P450, through metabolism to 13-hydroxyellipticine and ellipticine N2-oxide. Cancer Res. 64 (22), 8374-8380 (2004).
- Kotrbová, V., et al. Cytochrome b5 shifts oxidation of the anticancer drug ellipticine by cytochromes P450 1A1 and 1A2 from its detoxication to activation, thereby modulating its pharmacological efficacy. Biochem. Pharmacol. 82 (6), 669-680 (2011).
- Stiborová, M., et al. Cytochrome b5 increases cytochrome P450 3A4-mediated activation of anticancer drug ellipticine to 13-hydroxyellipticine whose covalent binding to DNA is elevated by sulfotransferases and N,O-acetyltransferases. Chem. Res.Toxicol. 2 (5), 1075-1085 (2012).
- Stiborová, M., et al. Ellipticine oxidation and DNA adduct formation in human hepatocytes is catalyzed by human cytochromes P450 and enhanced by cytochrome b5. Toxicology. 302 (2-3), 233-241 (2012).
- Sulc, M., et al. Effectiveness of human cytochrome P450 3A4 present in liposomal and microsomal nanoparticles in formation of covalent DNA adducts by ellipticine. Neuro Endocrinol. Lett. 37 (Suppl 1), 95-102 (2016).
- Stiborová, M., et al. Cytochrome b5 plays a dual role in the reaction cycle of cytochrome P450 3A4 during oxidation of the anticancer drug ellipticine. Monatsh. Chem. 148 (11), 1983-1991 (2017).
- Stiborová, M., Poljaková, J., Ryslavá, H., Dracínský, M., Eckschlager, T., Frei, E. Mammalian peroxidases activate anticancer drug ellipticine to intermediates forming deoxyguanosine adducts in DNA identical to those found in vivo and generated from 12-hydroxyellipticine and 13-hydroxyellipticine. Int. J. Cancer. 20 (2), 243-251 (2007).
- Enya, T., Suzuki, H., Watanabe, T., Hirayama, T., Hisamatsu, Y. 3-Nitrobenzanthrone, a powerful bacterial mutagen and suspected human carcinogen found in diesel exhausts and airborne particulates. Environ. Sci. Technol. 31 (10), 2772-2776 (1997).
- Seidel, A., Dahmann, D., Krekeler, H., Jacob, J. Biomonitoring of polycyclic aromatic compounds in the urine of mining workers occupationally exposed to diesel exhaust. Int. J. Hyg. Environ. Health. 204 (5-6), 333-338 (2002).
- Arlt, V. M. 3-Nitrobenzanthrone, a potential human cancer hazard in diesel exhaust and urban air pollution: a review of the evidence. Mutagenesis. 20 (6), 399-410 (2005).
- Hansen, T., Seidel, A., Borlak, J. The environmental carcinogen 3-nitrobenzanthrone and its main metabolite 3-aminobenzanthrone enhance formation of reactive oxygen intermediates in human A549 lung epithelial cells. Toxicol. Appl. Pharmacol. 221 (2), 222-234 (2007).
- Stiborová, M., et al. 3-aminobenzanthrone, a human metabolite of the carcinogenic environmental pollutant 3-nitrobenzanthrone, induces biotransformation enzymes in rat kidney and lung. Mutat. Res. 676 (1-2), 93-101 (2009).
- Nagy, E., et al. DNA adduct and tumor formations in rats after intratracheal administration of the urban air pollutant 3-nitrobenzanthrone. Carcinogenesis. 26 (10), 1821-1828 (2005).
- Stiborová, M., et al. The environmental pollutant and carcinogen 3-nitrobenzanthrone and its human metabolite 3-aminobenzanthrone are potent inducers of rat hepatic cytochromes P450 1A1 and -1A2 and NAD(P)H:quinone oxidoreductase. Drug Metab. Dispos. 34 (8), 1398-1405 (2006).
- Stiborová, M., et al. The environmental pollutant and carcinogen 3-nitrobenzanthrone induces cytochrome P450 1A1 and NAD(P)H:quinone oxidoreductase in rat lung and kidney, thereby enhancing its own genotoxicity. Toxicology. 247 (1), 11-22 (2008).
- Poirier, M. C. Chemical-induced DNA damage and human cancer risk. Nat. Rev. Cancer. 4 (8), 630-637 (2004).
- Poirier, M. C. Chemical-induced DNA damage and human cancer risk. Discov. Med. 14 (77), 283-288 (2012).
- Phillips, D. H. Detection of DNA modifications by the 32P-postlabelling assay. Mutat. Res. 378 (1-2), 1-12 (1997).
- Phillips, D. H., et al. Methods of DNA adduct determination and their application to testing compounds for genotoxicity. Environ. Mol. Mutagen. 35 (3), 222-233 (2000).
- Phillips, D. H. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 23 (12), 1979-2004 (2002).
- Phillips, D. H., Hewer, A., Arlt, V. M. 32P-postlabeling analysis of DNA adducts. Methods Mol. Biol. 291, 3-12 (2005).
- Farmer, P. B., et al. DNA adducts: mass spectrometry methods and future prospects. Toxicol. Appl. Pharmacol. 207 (2 Suppl), 293-301 (2005).
- Singh, R., Farmer, P. B. Liquid chromatography-electrospray ionization-mass spectrometry: the future of DNA adduct detection. Carcinogenesis. 27 (2), 178-196 (2006).
- Phillips, D. H., Arlt, V. M. The 32P-postlabeling assay for DNA adducts. Nat. Protoc. 2 (11), 2772-2781 (2007).
- Phillips, D. H. On the origins and development of the (32)P-postlabelling assay for carcinogen-DNA adducts. Cancer Lett. 334 (1), 5-9 (2013).
- Phillips, D. H., Arlt, V. M. 32P-postlabeling analysis of DNA adducts. Methods Mol. Biol. 1105, 127-138 (2014).
- Stiborová, M., Frei, E., Bieler, C. A., Schmeiser, H. H. 32P-Postlabelling: a sensitive technique for the detection of DNA adducts. Chem. Listy. 92, 661-668 (1998).
- Stiborová, M., Rupertová, M., Hodek, P., Frei, E., Schmeiser, H. H. Monitoring of DNA adducts in humans and 32P-postlabelling methods. A review. Collect. Czech. Chem. Commun. 69, 477-498 (2004).
- Beach, A. C., Gupta, R. C. Human biomonitoring and the 32P-postlabelling assay. Carcinogenesis. 13, 1053-1074 (1992).

