Processing of Human Cardiac Tissue Toward Extracellular Matrix Self-assembling Hydrogel for In Vitro and In Vivo Applications
Özet
This protocol describes the complete decellularization of human myocardium while preserving its extracellular matrix components. Further processing of the extracellular matrix results in the production of microparticles and a cytoprotective self-assembling hydrogel.
Abstract
Acellular extracellular matrix preparations are useful for studying cell-matrix interactions and facilitate regenerative cell therapy applications. Several commercial extracellular matrix products are available as hydrogels or membranes, but these do not possess tissue-specific biological activity. Because perfusion decellularization is usually not possible with human heart tissue, we developed a 3-step immersion decellularization process. Human myocardial slices procured during surgery are first treated with detergent-free hyperosmolar lysis buffer, followed by incubation with the ionic detergent, sodium dodecyl sulfate, and the process is completed by exploiting the intrinsic DNase activity of fetal bovine serum. This technique results in cell-free sheets of cardiac extracellular matrix with largely preserved fibrous tissue architecture and biopolymer composition, which were shown to provide specific environmental cues to cardiac cell populations and pluripotent stem cells. Cardiac extracellular matrix sheets can then be further processed into a microparticle powder without further chemical modification, or, via short-term pepsin digestion, into a self-assembling cardiac extracellular matrix hydrogel with preserved bioactivity.
Introduction
The extracellular matrix (ECM) provides not only structural support but is also important for biologic cell and tissue function1. In the heart, the ECM participates in the regulation of pathophysiologic responses such as fibrosis, inflammation, angiogenesis, cardiomyocyte contractile function and viability, and resident progenitor cell fate. In addition to its primary components – fibrous glycoproteins, glycosaminoglycans, and proteoglycans – it contains a host of secreted growth factors, cytokines, and membranous vesicles containing nucleic acids and proteins2,3.
It has recently become clear that acellular ECM preparations are not only invaluable for studying cell-matrix interactions, but also for potential therapeutic cell-based applications. The importance of providing an adequate environment to therapeutic cell products or engineered tissues is now widely acknowledged. Attempts have been made to combine cell suspensions or active compounds with defined biopolymeric hydrogels4,5,6 or with protein cocktails secreted by murine sarcoma cells (i.e., Matrigel, Geltrex)7. However, the former have limited bioactivity, the latter are problematic in GMP-grade processes, and both lack the tissue-specific bioactivity of cardiac ECM (cECM)8,9,10,11,12,13.
Decellularization of the myocardium has previously been performed by perfusion of the whole heart via the coronary vasculature14,15. While this is possible in animal hearts, intact human hearts are rarely available. Therefore, an immersion process that allows for handling tissue samples obtained in the operating room was favored. Our "3-step" protocol contains 3 separate incubation steps namely lysis, solubilization, and DNA removal. It yields human myocardial ECM with largely preserved protein and glycosaminoglycan composition16,17. These cECM slices allow for in vitro studies of cell-matrix interactions but are poorly suited for potential human-scale therapeutic applications. The manufacturing process was then extended to produce either lyophilized cECM microparticles or a cECM hydrogel18.
This protocol allows for the decellularization of human myocardium obtained from surgical samples, preserving the major components of myocardial extracellular matrix (ECM) and their biologic activity. This protocol is recommended when human cardiac ECM with preserved tissue-specific bioactivity is required for experimental studies of cell-matrix interactions, or when a suitable environment is needed for cell-based myocardial regeneration approaches. In principle, it is also possible to adapt this protocol to GMP-grade conditions, so that the use of processed cECM should be feasible in future therapeutic applications.
Protocol
The study protocol conforms to the ethical principles outlined in the Declaration of Helsinki and was approved by the institutional review board and ethics committee of Charité Medical University. All patients provided written, informed consent for use of heart tissue for experimental studies.
1. Preparation of Human Myocardium for Sectioning
- Obtain left ventricular myocardium (size varies depending on the type of surgery) directly from the operating room and transport in cold PBS in a sterile container.
- Remove all fat tissue with a sterile scalpel with a no. 10 blade under sterile conditions.
- Cut the myocardium in cubes of approximately 1 x 1 x 1 cm using a scalpel.
- Place the cubes in sterile 50 mL tubes.
NOTE: To pause, perform step 1.5, otherwise continue with step 2.2. Avoid repeated freeze-thaw cycles to prevent protein degradation and a decrease in tissue quality. - Store the tubes containing the cubes at -80 °C. The tissue can be stored for several months.
2. Sectioning of the Myocardium Cubes into Slices
- Take the tube(s) containing the prepared cubes out of the freezer.
- Transport on ice to the cryostat.
- Set the object and chamber temperature to -15 °C.
NOTE: The right temperature is crucial to guarantee perfect sectioning. - Chill empty 50 mL tubes and stamps in the chamber or on dry ice.
- Add a layer (approximately 0.5 – 1.0 mL) of cryosection medium onto the cold stamp and let it freeze until it is white and solid.
- Add a second layer of cryosection medium and place a myocardium cube onto this layer.
- Make sure the cryosection medium and myocardium are completely frozen before you continue.
NOTE: When the sample and cryosection medium are not frozen the sectioning will be impaired. Completely frozen cryosection medium turns from fluid and transparent to solid, white, and non-transparent. The myocardial cubes need at least 30 min up to 1 h to completely freeze if continuing immediately from step 1.4. When the tissue is frozen it should not deform when touched/handled with tweezers. - Insert stamp with frozen myocardium into the holder and tighten all screws.
- Trim the surface of the tissue until an even sectioning surface is obtained.
- Set sectioning thickness to 300 µm.
- Section the frozen myocardium with automatic sectioning. This guarantees uniform cut sections. Approximately 30 – 40 sections can be sliced from one cube.
- Place each slice after sectioning loosely in a precooled 50 mL tube.
NOTE: Do not press the slices or pack tightly. Approximately 40 slices can fit in one tube. - Close the filled tubes and place on ice.
NOTE: To pause go on with step 2.14, otherwise continue with step 3.2. - Store myocardium slices at -80 °C. The slices can be stored for several months.
3. Decellularization of Human Myocardium Slices
- Take the necessary number of 50 mL tubes containing myocardium sections out of the -80 °C freezer and transport on ice.
- Transfer the frozen sections of all 50 mL tubes from step 3.1 into one sterile beaker. Approximately 40 slices are added to the beaker per 50 mL tube. The beaker should be three times the volume of the 50 mL tubes; for example, for decellularization of one 50 mL tube use a 150 mL beaker.
- Add 50 mL lysis solution (10 mM Tris, 0.1% w/v EDTA, pH 7.4 in H2O) per 50 mL tube with myocardium slices.
- Shake the myocardium slices in a sterile beaker at 100 – 150 rpm continuously for 2 h at room temperature.
- Strain the liquid with the tissue slices through a coarse strainer. Transfer the tissue slices with a blunt tweezer to a new sterile beaker. Add 50 mL of 0.5% SDS in PBS per initial 50 mL tube of myocardium slices (e.g., use 100 mL SDS solution when two 50 mL tubes of myocardial sections are being decellularized).
- Shake continuously for 6 h at 100 – 150 rpm at room temperature.
- Strain the liquid with the tissue slices through a coarse strainer. Transfer the tissue slices with a blunt tweezer to a new sterile beaker and wash 3 times with 50 mL PBS for 10 min each while shaking at 150 rpm. Next, wash overnight in PBS with 1% Penicillin/Streptomycin and 1% Nystatin (PBS-P/S-N) at 100-150 rpm and 4 °C while shaking.
NOTE: Thorough washing is crucial to completely remove any residual SDS. Remaining traces of SDS are toxic when the ECM is used in combination with cells. - Remove the washing solution and add 25 mL preheated FBS (37 °C) with 1% Penicillin/Streptomycin and 1% Nystatin to the decellularized slices per initial 50 mL tube of myocardium slices.
- Incubate for 3 h at 37 °C. In this step, any remaining DNA is removed from the matrix due to the intrinsic DNase activity of the FBS.
- Transfer the slices to a new sterile beaker and wash 3 times with 50 mL PBS for 10 min each while shaking at 150 rpm.
NOTE: The ECM slices can be stored in PBS-P/S-N at 4 °C for several days. However, it is recommended to proceed immediately.
4. Processing the Decellularized cECM to a Powder
- Place the ECM slices loosely in a new sterile 6-well plate. Freeze the ECM at -80 °C and lyophilize for 2 days in a lyophilizer.
NOTE: More than one slice can be placed per well. Arrange the slices to completely cover the surface of the well. Overlapping the slices does not affect the subsequent pulverization success. - For the pulverization of the ECM slice, use specific milling tubes (see Table of Materials) containing 1.4 mm ceramic beads.Fill the tubes loosely with lyophilized ECM using sterile blunt tweezers. For optimal results, a total weight of ECM between 10 – 20 mg is recommended.
NOTE: If the ECM is packed too tightly in the milling tubes the pulverization process will be negatively affected. - Snap-freeze the tubes in liquid nitrogen.
CAUTION: Liquid nitrogen is extremely low temperature, wear appropriate personal protection. - Insert the frozen tubes into the milling machine and pulverize the ECM.
- Set a duration of 30 s and a maximum speed and start the device.
- After finishing, take out the tubes and snap-freeze again in liquid nitrogen.
- Repeat five times.
- Wash the microparticles from the tubes by adding 1 mL ddH2O per tube and shake or vortex for complete solubilizing. Filter the heterogeneous solution through a 200 µm mesh into a 50 mL tube.
- Freeze the tube in -80 °C or liquid nitrogen.
- Replace the standard lid of the tube with a 0.22 µm filter. This filter lid prevents the powder from flowing out of the tube but allows air circulation for water evaporation.
- Insert the tubes in the lyophilizer and lyophilize again to remove the water from the step (step 4.5)
NOTE: Lyophilization at this step can take up to 3 days. - Store powder at -80 °C until further processing or continue with step 5.1.
5. Enzyme Based Homogenizing of cECM Micro-Particles
- Dissolve porcine pepsin in 0.01M HCl (pH 2.0) to a concentration of 1 mg/mL. Place on a rotary shaker at room temperature until it is completely dissolved.
- Dissolve the lyophilized human ECM powder (Step 4.8) in the pepsin solution to a final concentration of 10 mg/mL. Mix thoroughly through pipetting. The total volume depends on the amount of ECM solution needed. For example, dissolve 10 mg of ECM powder in 1 mL pepsin solution.
NOTE: Dissolving particles can be supported through gentle vortexing (1,000 – 1,500 RPM). Remaining lumps of microparticles will negatively affect the digestion. - Transfer the solution to 2 mL tubes with a volume of 1 mL per tube.
- Place the tubes in a tube shaker for 48 h at 27 °C and 1,200 rpm.
- Take the tubes out of the shaker and place on ice or a pre-cooled tube holder.
- Mix 1/9 of the volume of ECM solution of cold 10x PBS (pH 7.4) with 1/10 of the volume of ECM solution of cold 0.1M NaOH in a precooled tube and place on ice. This mixture will neutralize the digested ECM solution and irreversibly inactivate the pepsin.
- Add the ECM solution to the neutralization mixture and mix thoroughly through pipetting or vortexing.
- Set the ECM to the desired concentration with 1x PBS (pH 7.4). The pH of the solution can be checked with pH paper.
NOTE: Example calculation for 10 mL digested ECM solution:
Add 10 mg porcine pepsin to 10 mL HCl (pH 2.0).
Add 100 mg ECM powder to pepsin solution.
NOTE: Example calculation for neutralizing 10 mL digested ECM solution and setting the concentration to 8 mg/mL:
VolFinal = cstart x Volstart / cFinal = 10 mg/mL x 10 mL / 8 mg/mL = 12.5 mL
Vol10xPBS = Volstart / 9 = 1/9 from 10 mL = 1.11 mL 10x PBS
VolNaOH = Volstart / 10 = 1/10 from 10 mL = 1 mL
Vol1xPBS = VolFinal – Volstart – Vol10xPBS – VolNaOH = 12.5 mL – 10 mL – .11 mL – 1 mL = 0.39 mL
Representative Results
The 3-step protocol for decellularization of human myocardium presented here results in near-complete removal of cellular material, while preserving the key ECM components and the fibrillar structure of ECM. After decellularization, the gross removal of cells from the tissue is evident by the change in color (Figure 1A). Histological analysis with H&E and Masson Trichrome stains revealed the complete absence of residual intact cells (Figure 1B). Quantitative assays for individual ECM components revealed a more complete DNA removal and better preservation of total collagen, elastin, and glycosaminoglycans as compared with decellularization by SDS alone (Figure 2A). Functional evidence of the biologic activity of cECM is shown in Figure 2B. Here, RT-PCR was used to quantify the expression of structural cardiomyocyte proteins (Myh6, Tnnt2), early (Mef2c, Nkx2.5) and late (Gata4) cardiomyogenic transcription factors, and endothelial cell surface marker genes (von Willibrand Factor (vWF), VE-cadherin) in murine ESC undergoing spontaneous differentiation. Compared to both uncoated culture dish surface and Matrigel-ECM coating, it is evident that contact with cECM drives pluripotent stem cells preferably towards a cardiomyocyte-like phenotype.
Lyophilized ECM slices can be further processed into microparticles without using enzymatic or chemical reagents. Mechanical grinding or pulverizing using suitable kits allowed for production of microparticles with a uniform size (Figure 3A). Mass spectrometry provides an overview of all recognizable proteins present in cECM. Gene ontology analysis concentrating on cellular components revealed that the majority of ECM proteins are indeed derived from extracellular space, with few hemoglobin residues and a number of predominantly intracellular proteins (Figure 4). This pattern may determine the tissue-specific biologic function of cECM, but this hypothesis needs further research. A summary of the cECM protein composition is shown in Table 1. Processing the ECM into microparticles preserves its biological activity. HL-1 cells exposed to simulated "ischemic" conditions (hypoxia, glucose and serum deprivation) displayed an increase in cell metabolism (Figure 3B) and a decrease in cell death when cultured on cECM (Figure 3C).
Limited pepsin-based digestion of the cECM powder, based on a modified protocol developed for collagen purification19, results in a homogenized ECM solution. Phase contrast light microscopy pictures (Figure 5A) demonstrate the digestion process after 0 h and 48 h. This homogenizing step facilitates applications in regenerative medicine10. The resulting processed cECM is shown in Figure 5B. Below room temperature it remains liquid (Figure 5B, left) but at 37 °C it forms a self-assembling hydrogel with stability suitable for an overlay with cell suspensions allowing 3D culture (representative picture, Figure 5B right, hydrogel layered with culture medium). Live/dead staining of human cardiac fibroblasts also shows the beneficial effects of culturing on cECM (Figure 5C). Both cECM microparticles and cECM hydrogel enhanced the metabolic activity of contractile HL1 cells in ischemic conditions as compared to cells in standard culture (Figure 5C, lower right panel).
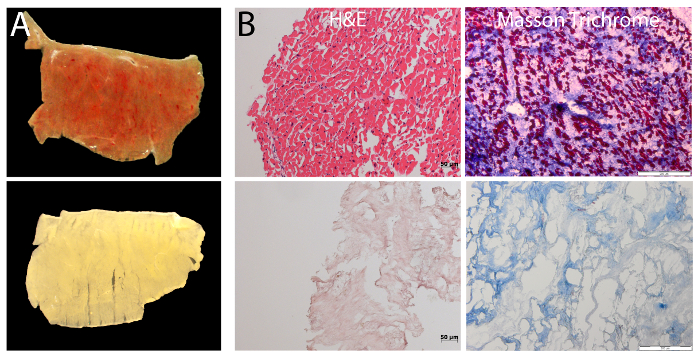
Figure 1: Decellularization of human cardiac myocardium. (A) Macroscopic analysis of cECM by stereo microscopy reveals the removal of cellular material with the 3-step decellularization protocol. This is evident by the increase in transparency. (B) Histology staining of myocardial slices before and after decellularization with HE- (left; Scale bar = 50 µm) and Masson Trichrome staining (right; Scale bar = 200 µm) shows successful removal of cells from the tissue. Please click here to view a larger version of this figure.
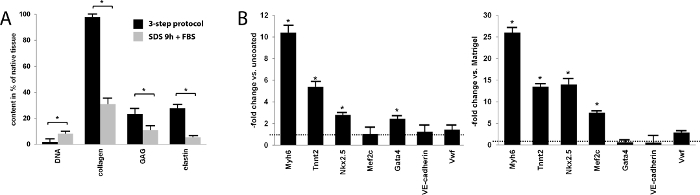
Figure 2: Content and effect of cECM slices. (A) Quantitative biochemical analysis of selected ECM components and DNA, comparing the 3-step decellularization protocol to 0.5% SDS-alone decellularization. Data are expressed as percentage of the content in native myocardium. With the 3-step protocol, the residual DNA content was nearly zero and significantly lower than that after a 9 h incubation with a standard combined 0.5% SDS/lysis buffer combination and FBS (SDS 9 h + FBS). Respective biochemical assays demonstrated a near-complete preservation of collagen, approximately 20% preservation of glycosaminoglycan content compared to native tissue, and a nearly 30% preservation of elastin content, all significantly higher than with the standard SDS reference protocol (Bars represent mean ± SEM). (B) mRNA expression of selected genes in induced pluripotent stem cells differentiating on cECM, compared to uncoated culture dishes and to Matrigel-coated surface. cECM data are normalized to those obtained on the respective control materials (control = 1, dashed line). The majority of cardiac genes are significantly more highly expressed when cultured on cECM (Bars represent mean ± SEM). Please click here to view a larger version of this figure.
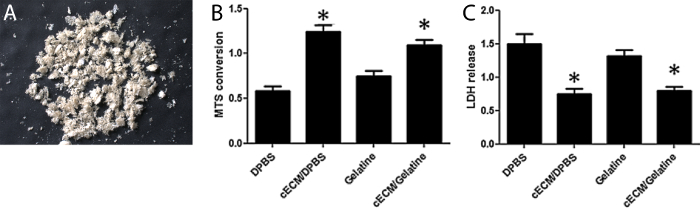
Figure 3: Appearance and biological activity of cECM microparticles. (A) cECM microparticle powder after lyophilization. (B) Metabolism (MTS) and (C) Cell death (LDH release) analysis of HL-1 cells cultured with cECM or gelatin. cECM significantly reduces cell death and increases metabolism. Bars represent mean ± SEM, statistical significance tested by one-way ANOVA and Bonferroni. Figure 3B-C have been modified from Kappler et al.18 Please click here to view a larger version of this figure.
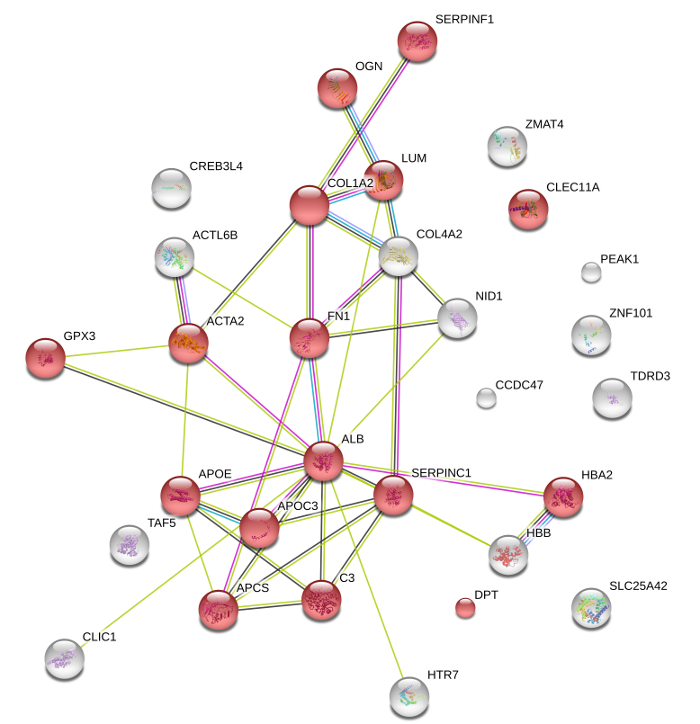
Figure 4: Protein composition of cECM microparticles. Following mass spectrometry, String DB analysis depicts the key components of cECM, which are mainly associated with the extracellular space (GO:0005615; red spheres p >0.05). Lines indicate protein interactions based on experimental-biochemical data (purple), on co-expression data (black), on database interactions (blue), and on co-expression (green). String DB required confidence: score >0.4. Please click here to view a larger version of this figure.
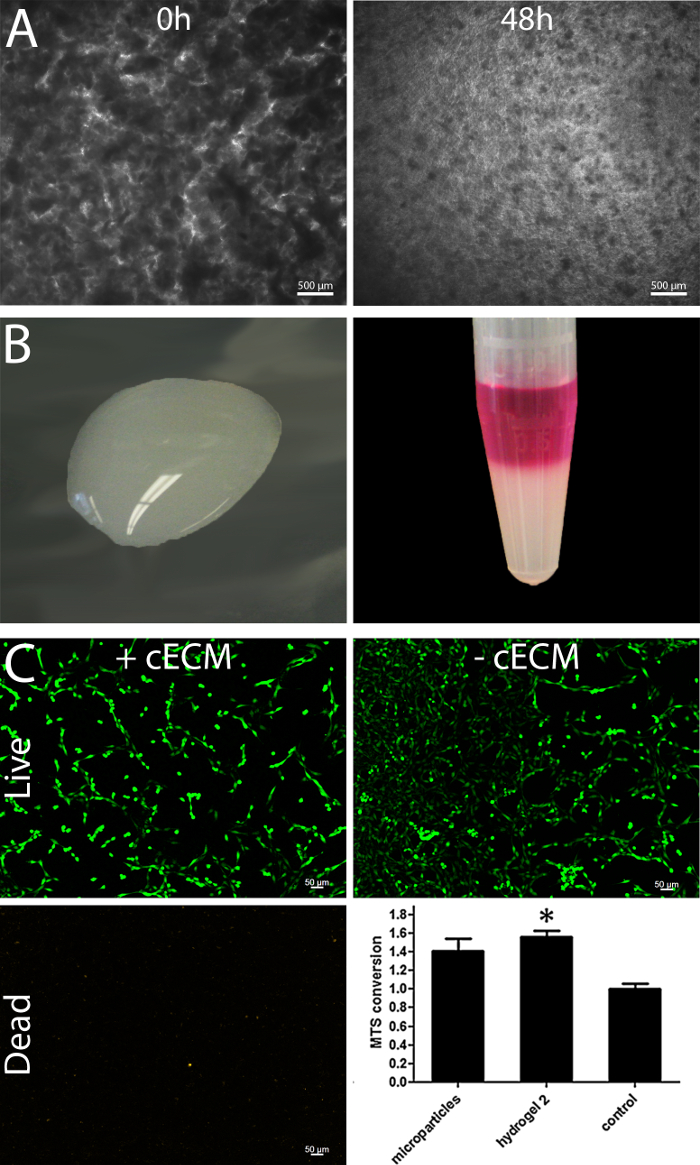
Figure 5: Processing of cECM to a hydrogel preserve the biological effectivity. Phase contrast light microscopy pictures demonstrate that enzymatic digestion of the cECM for 48 h results in a more homogenized ECM solution; Scale bar = 500 µm. (A) The ECM solution remains liquid up to room temperature (B, left) and contains the potential to self-assemble towards a hydrogel when incubated at 37 °C (B, right). Live/dead stain of cardiac human fibroblasts cultured with and without cECM reveals a higher calcein staining intensity of living cells; Scale bar = 50 µm (C). The cECM hydrogel increases the metabolic activity of HL1 cells in ischemic conditions in the same manner as the micro particles (C, lower right panel, modified from Kappler et al.18) Bars represent mean ± SEM, statistical significance tested by one-way ANOVA and Bonferroni. Please click here to view a larger version of this figure.
| Protein Description |
| 5-hydroxytryptamine receptor 7 OS=Homo sapiens GN=HTR7 PE=1 SV=2 |
| Actin-like protein 6B OS=Homo sapiens GN=ACTL6B PE=1 SV=1 |
| Actin, aortic smooth muscle OS=Homo sapiens GN=ACTA2 PE=1 SV=1 |
| Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 |
| Antithrombin-III OS=Homo sapiens GN=SERPINC1 PE=1 SV=1 |
| Apolipoprotein C-III OS=Homo sapiens GN=APOC3 PE=1 SV=1 |
| Apolipoprotein E OS=Homo sapiens GN=APOE PE=1 SV=1 |
| Coiled-coil domain-containing protein 47 OS=Homo sapiens GN=CCDC47 PE=1 SV=1 |
| C-type lectin domain family 11 member A OS=Homo sapiens GN=CLEC11A PE=1 SV=1 |
| Chloride intracellular channel protein 1 OS=Homo sapiens GN=CLIC1 PE=1 SV=4 |
| Collagen alpha-2(I) chain OS=Homo sapiens GN=COL1A2 PE=1 SV=7 |
| Complement C3 OS=Homo sapiens GN=C3 PE=1 SV=2 |
| Collagen alpha-2(IV) chain OS=Homo sapiens GN=COL4A2 PE=1 SV=4 |
| Cyclic AMP-responsive element-binding protein 3-like protein 4 OS=Homo sapiens GN=CREB3L4 PE=1 SV=1 |
| Dermatopontin OS=Homo sapiens GN=DPT PE=2 SV=2 |
| Fibronectin OS=Homo sapiens GN=FN1 PE=1 SV=4 |
| Glutathione peroxidase 3 OS=Homo sapiens GN=GPX3 PE=1 SV=2 |
| Hemoglobin subunit alpha OS=Homo sapiens GN=HBA1 PE=1 SV=2 |
| Hemoglobin subunit beta OS=Homo sapiens GN=HBB PE=1 SV=2 |
| Ig kappa chain C region OS=Homo sapiens GN=IGKC PE=1 SV=1 |
| Lumican OS=Homo sapiens GN=LUM PE=1 SV=2 |
| Mimecan OS=Homo sapiens GN=OGN PE=1 SV=1 |
| Nidogen-1 OS=Homo sapiens GN=NID1 PE=1 SV=3 |
| Pseudopodium-enriched atypical kinase 1 OS=Homo sapiens GN=PEAK1 PE=1 SV=4 |
| Pigment epithelium-derived factor OS=Homo sapiens GN=SERPINF1 PE=1 SV=4 |
| Mitochondrial coenzyme A transporter SLC25A42 OS=Homo sapiens GN=SLC25A42 PE=2 SV=2 |
| Serum amyloid P-component OS=Homo sapiens GN=APCS PE=1 SV=2 |
| Transcription initiation factor TFIID subunit 5 OS=Homo sapiens GN=TAF5 PE=1 SV=3 |
| Tudor domain-containing protein 3 OS=Homo sapiens GN=TDRD3 PE=1 SV=1 |
| Zinc finger matrin-type protein 4 OS=Homo sapiens GN=ZMAT4 PE=2 SV=1 |
| Zinc finger protein 101 OS=Homo sapiens GN=ZNF101 PE=2 SV=1 |
Table 1: Protein composition of cECM powder as determined by mass spectrometry.
Discussion
When preparing human myocardial ECM, the goal is to achieve the following: removal of relevant immunogenic cellular material, preservation of ECM integrity and bioactivity, sterility, non-toxicity of the end-product, GMP-process compatibility, and suitability of the product for a given application in terms of handling. By combining our 3-step decellularization protocol with further processing into microparticles or self-assembling hydrogel, human cardiac ECM material is obtained that possesses specific biological activity, is easy to handle, and has the potential for multiple applications in vitro and in vivo, similar to what has been shown for ECM products from several animals species11,13,20,21.
During the initial processing of the myocardial tissue, it is important to section myocardial slices of identical thickness, because compound concentration and treatment duration have been titrated for 300 µm slices. Thicker slices will be incompletely decellularized and thinner slices will suffer unwanted breakdown of ECM components with loss of bioactivity. This is particularly important with regards to the SDS treatment, where toxicity can negatively affect ECM properties and cellular analyses16. Furthermore, prolonged SDS treatment can lead to complete absence of fibronectin as seen in the study by Godier-Fournement et al.22, while it is preserved with our method17. The cECM sections are suitable for use in experiments without further processing (i.e., in 3D culture models analyzing cell differentiation or cell-ECM interactions, or for "tissue engineering" cellular repopulation approaches). The use of FBS for DNA removal is highly efficient, but could potentially lead to some residual FBS proteins in the ECM sections. This could influence any FBS sensitive assays. The translation to a clinical grade GMP protocol should be possible with the use of certified sera, however an adaptation might be necessary depending on regulations. This could either be confirming the absence of FBS residues in the ECM or an alteration to the protocol will be necessary to replace the FBS with DNase treatment. Lastly, repeated freeze-thaw cycles of the tissue and ECM sections are to be avoided to prevent degradation of the matrix.
When producing the cECM hydrogel, pepsin digestion is the most critical step in the process. In our initial experiments, pepsin digestion at pH 1 for 48 h at 37 °C led to a loss of bioactivity, which could be prevented by modifying the incubation to pH 2 for 48 h at 27 oC. Also, bear in mind that the hydrogel is produced from lyophilized cECM microparticle suspension, not from freshly decellularized wet cECM slices. In addition to the improved general handling of the hydrogel as compared to cECM slices, the hydrogel has the advantage that it can be diluted for use in different concentrations, for instance at a higher concentration in combination with other materials or for 3D culture, or at a lower concentration for coating of cell culture dishes or as an additive to culture medium.
This protocol has been established as a laboratory-grade SOP for human cECM preparation to help fill the gaps in translational regenerative medicine that were recently described by Saldin et al.10 While decellularized cECM slices may be applied as sheets on the epicardial surface of the diseased heart, as recently described by Sarig et al., with porcine cECM in a rat model of myocardial infarction23, processing into a hydrogel allows for more diverse in vivo applications, such as the injection of cECM into myocardium as shown by Singelyn et al.13,24 In order to proceed toward clinical translational activities, the current protocol will have to be converted to GMP-grade procedures. In principle, all materials used are also part of approved medical device/ATMP production processes (i.e., decellularized heart valves or blood vessels) and no major obstacles are to be expected. However, information on the safe storage duration and absence of pathogens will certainly be required. Ultimately, a reliable source of larger quantities of fresh and sterile heart tissue from donors without heart disease is needed. Here, hearts from deceased organ donors that are not suitable for transplantation are the most likely scenario.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
The study protocol conforms to the ethical principles outlined in the Declaration of Helsinki. Patients provided informed consent for the use of the tissue for research purposes, and the process of tissue collection was approved by the Institutional Review Board and ethics committee of Charité – Universitätsmedizin Berlin (EA4/028/12).
Materials
| Balance | DR Precisa, Dietikon, Switzerland | Precisa XR 205SM | |
| Blades Nr.10 Skalpell Nr.3 | InstrumenteNRW, Erftstadt, Germany | SK-10-004 | |
| Cell culture plates (6-well) | Greiner, Frickenhausen, Germany | 657160 | |
| Cryostat CM | Leica, Wetzlar, Germany | 3050S | |
| EDTA | Carl Roth, Karlsruhe, Germany | 8043.3 | |
| Eppendorf reaction tubes (1.5 or 2 ml) | Greiner, Frickenhausen, Germany | 616201, 623201 | |
| Falcon 15ml, 50ml | Greiner, Frickenhausen, Germany | 188271, 227270 | |
| Fetal Bovine Serum (FBS) | Biochrome, Berlin, Germany | S 0115 | |
| Freeze Dry System | Labconco, Kansas City, USA | 7670520 | |
| Freezer (-80°C) | Thermo Scientific, Waltham, MA, USA | Forma 900 Series | |
| HCl | Carl Roth, Karlsruhe, Germany | 281.1 | |
| Microtome Blades Type 819 | Leica, Wetzlar, Germany | 14035838925 | |
| Minilys Homogeniser | PEQLAB Biotechnologie GmbH, Erlangen, Germany | 91-PCSM | |
| NaOH | Carl Roth, Karlsruhe, Germany | K021.1 | |
| Nystatin | PAN Biotech, Aidenbach, Germany | P06-07800 | |
| PBS | Thermo Scientific, Waltham, MA, USA | 14190-094 | |
| Penicillin/streptomycin | Life Technologies, Darmstadt, Germany | 15140122 | |
| Pepsin | Sigma-Aldrich, Taufkirchen, Germany | P6887-1G | |
| Precellys Keramik-Kit 1.4 mm | Peqlab Biotechnolgie, Erlangen, Germany | 91-PCS-CK14 | |
| Rotamax 120 Plate shaker | Heidolph, Schwabach, Germany | 544-41200-00 | |
| SDS | Carl Roth, Karlsruhe, Germany | CN30.3 | |
| Stereo microscope | Leica, Wetzlar, Germany | M125 | |
| Steriflip-GP, 0,22 µm | Merck Millipore, Darmstadt, Germany | SCGP00525 | |
| Stuart analogue rocker & roller mixers | Sigma-Aldrich, Taufkirchen, Germany | Z675113-1EA | |
| Tissue Tek O.C.T compound | Hartenstein, Wurzburg, Germany | TTEK | |
| Transfusion set 200µm | Sarstedt, Nümbrecht, Germany | 798.200.500 | |
| TRIS | Carl Roth, Karlsruhe, Germany | 5429.3 | |
| vedena Skalpellgriff Fig. 3, Standard, 125 mm | Medical Highlights, Rohrdorf, Germany | CV102-003 | |
| Vortex-Genie2 | Scientific Industry, New York, USA | SI-0256 |
Referanslar
- Elliott, R., Hoehn, J. Use of Commercial Porcine Skin for Wound Dressings. Plastic and reconstructive surgery. 52 (4), 401-405 (1973).
- Rienks, M., Papageorgiou, A. -. P., Frangogiannis, N. G., Heymans, S. Myocardial Extracellular Matrix: An Ever-Changing and Diverse Entity. Circulation Research. 114 (5), 872-888 (2014).
- Prabhu, S. D., Frangogiannis, N. G. The Biological Basis for Cardiac Repair After Myocardial Infarction. Circulation Research. 119 (1), 91-112 (2016).
- Boopathy, A. V., Martinez, M. D., Smith, A. W., Brown, M. E., Garcia, A. J., Davis, M. Intramyocardial Delivery of Notch Ligand-Containing Hydrogels Improves Cardiac Function and Angiogenesis Following Infarction. Tissue Eng Part A. 21 (17-18), 2315-2322 (2015).
- Gaetani, R., Yin, C., et al. Cardiac derived extracellular matrix enhances cardiogenic properties of human cardiac progenitor cells. Cell Transplant. , (2015).
- Kraehenbuehl, T. P., Ferreira, L. S., et al. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 32 (4), 1102-1109 (2011).
- Zhang, J., Klos, M., et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: The matrix sandwich method. Circulation Research. 111 (9), 1125-1136 (2012).
- Fong, A. H., Romero-López, M., et al. Three-Dimensional Adult Cardiac Extracellular Matrix Promotes Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Tissue Engineering Part A. 22 (15-16), 1016-1025 (2016).
- DeQuach, J. A., Mezzano, V., et al. Simple and High Yielding Method for Preparing Tissue Specific Extracellular Matrix Coatings for Cell Culture. PLoS ONE. 5 (9), e13039 (2010).
- Saldin, L. T., Cramer, M. C., Velankar, S. S., White, L. J., Badylak, S. F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomaterialia. 49, 1-15 (2017).
- Tukmachev, D., Forostyak, S., et al. Injectable extracellular matrix hydrogels as scaffolds for spinal cord injury repair. Tissue Eng Part A. , (2016).
- Freytes, D. O., Martin, J., Velankar, S. S., Lee, A. S., Badylak, S. F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 29 (11), 1630-1637 (2008).
- Singelyn, J. M., Sundaramurthy, P., et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. Journal of the American College of Cardiology. 59 (8), 751-763 (2012).
- Wainwright, J. M., Czajka, C. A., et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 16 (3), 525-532 (2010).
- Ott, H. C., Matthiesen, T. S., et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nature Medicine. 14, 213-221 (2008).
- Oberwallner, B., Anic, B. A., et al. Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. Eur J Cardiothorac Surg. 47, 416-425 (2015).
- Oberwallner, B., Brodarac, A., et al. Preparation of cardiac extracellular matrix scaffolds by decellularization of human myocardium. Journal of Biomedical Materials Research Part A. 102 (9), 3263-3272 (2014).
- Kappler, B., Anic, P., et al. The cytoprotective capacity of processed human cardiac extracellular matrix. Journal of Materials Science: Materials in Medicine. , (2016).
- Bashey, R. I., Martinez-Hernandez, A., Jimenez, S. A. Isolation, characterization, and localization of cardiac collagen type VI. Associations with other extracellular matrix components. Circulation Research. 70 (5), (1992).
- Wu, J., Ravikumar, P., Nguyen, K. T., Hsia, C. C. W., Hong, Y., Gorler, A. Lung protection by inhalation of exogenous solubilized extracellular matrix. PLOS ONE. 12 (2), e0171165 (2017).
- Chen, W. C. W., Wang, Z., et al. Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Science Advances. 2 (11), e1600844 (2016).
- Godier-Furnémont, A. F. G., Martens, T. P., et al. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proceedings of the National Academy of Sciences of the United States of America. 108 (19), 7974-7979 (2011).
- Sarig, U., Sarig, H., et al. Natural myocardial ECM patch drives cardiac progenitor based restoration even after scarring. Acta Biomaterialia. 44, 209-220 (2016).
- Singelyn, J. M., DeQuach, J. A., Seif-Naraghi, S. B., Littlefield, R. B., Schup-Magoffin, P. J., Christman, K. L. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 30 (29), 5409-5416 (2009).

