Desensitization and Recovery of Crayfish Photoreceptors Upon Delivery of a Light Stimulus
Özet
A protocol for the study of desensitization and sensitivity recovery of crayfish photoreceptors as a function of circadian time is presented.
Abstract
A method to study desensitization and recovery of crayfish photoreceptors is presented. We performed intracellular electrical recordings of photoreceptor cells in isolated eyestalks using the discontinuous single electrode-switched voltage-clamp configuration. First, with a razor blade we made an opening in the dorsal cornea to get access to the retina. Thereafter, we inserted a glass electrode through the opening, and penetrated a cell as reported by the recording of a negative potential. Membrane potential was clamped at the photoreceptor’s resting potential and a light-pulse was applied to activate currents. Finally, the two light-flash protocol was employed to measure current desensitization and recovery. The first light-flash triggers, after a lag period, the transduction ionic current, which after reaching a peak amplitude decays towards a desensitized state; the second flash, applied at varying time intervals, assesses the state of the light-activated conductance. To characterize the light-elicited current, three parameters were measured: 1) latency (the time elapsed between light flash delivery and the moment in which current achieves 10% of its maximum value); 2) peak current; and 3) desensitization time constant (exponential time constant of the current decay phase). All parameters are affected by the first pulse.
To quantify recovery from desensitization, the ratio p2/p1 was employed versus time between pulses. p1 is the peak current evoked by the first light-pulse, and p2 is the peak current evoked by the second pulse. These data were fitted to a sum of exponential functions. Finally, these measurements were carried out as function of circadian time.
Introduction
In order to be perceived as a visual stimulus, light reaching the eyes must be transduced into an electrical signal. Hence, in all visual organisms, light triggers a transduction ion-current, which in turn produces a change in the membrane potential of photoreceptor cells, the so-called receptor potential. Due to this, the light sensitivity of the eye primarily depends on the state of the light activated conductance, which can be either available to be activated or desensitized.
In crayfish photoreceptors, light triggers a slow, transient, ionic current1. Upon illumination, the transduction current arises after a lag or latency before reaching its maximum; thereafter it decays, as the transduction channels fall into a desensitized state in which they are unresponsive to further light stimulus2. That is, light, in addition to activating the transduction current responsible of vision, also induces a transient decrement of the sensitivity of photoreceptor cells. Desensitization may represent a general protective mechanism against overexposure to an adequate stimulus. The eye's sensitivity to light is recovered as the transduction conductance recovers from desensitization.
Intracellular recording is a useful technique for measuring electrical activity of excitable cells3,4,5,6,7,8. Although intracellular recording has become less frequent with the advent of the patch-clamp technique9, it is still a convenient approach when cells are either difficult to isolate, or present a geometry that makes the formation of the patch-clamping giga-seals difficult (i.e., seals or tight-contacts between the patch electrode and membranes with electrical resistance of the order of 109ohms). Examples of the latter are sperm cells10 and the photoreceptor cells herein studied. In our experience, Procambarus clarkii photoreceptors are difficult to isolate and keep in primary culture; additionally, they are thin rods that make giga-seal formation difficult to achieve. In intracellular recordings, a sharp electrode is advanced into a cell that is kept in place by the surrounding tissue. The electrode is chopped by the high-speed switching circuitry of the amplifier, so current is sampled between voltage pulses. This mode is known as discontinuous single-electrode voltage clamp (dSEVC mode)11. The high resistance (small opening) of the electrode hinders the diffusional exchange between the cell and the pipette solutions, yielding a minimal disturbance of the intracellular milieu3. A potential drawback of this technique is that electrode insertion may produce a non-selective leak current; therefore, care must be taken to avoid recording from cells where the size of the leak current may interfere with the intended measurements4,12.
Herein, we use isolated crayfish eyestalks to assess desensitization and recovery of the light-activated ion conductance by performing intracellular electrical recordings of photoreceptor cells under voltage clamp conditions.
Protocol
NOTE: The experiments comply with the Laws of Animal Protection of Mexico.
1. Experimental Setup
- General connections
- Connect the amplifier to a suitable computer through an analog-to-digital converter and use an oscilloscope to monitor the experiment (Figure 1).
- Connect the photostimulator to the A/D converted.
- Recording chamber
- Place the recording chamber on top of an anti-vibration table and locate it inside a Faraday cage.
NOTE: This prevents mechanical vibration and electrical noise that may affect the recording. Our Faraday cage was made of darkened wire mesh. A homemade, 2 mL, acrylic recording chamber is used (Figure 2). - Prepare the bath solution to keep the preparation alive13: 205 mM NaCl; 5 mM KCl; 2 mM MgSO4; 13 mM CaCl2; 5 mM Hepes-NaOH, pH 7.3.
NOTE: Solution does not include dextrose or any bubbling by 95% O2, 5% CO2 mixture because the experimental procedure is short enough to detect damage by the electrical recording (see step 4.2.3 for completeness).
- Place the recording chamber on top of an anti-vibration table and locate it inside a Faraday cage.
- Electrodes
- Use a chloride-coated silver-wire electrode as the reference electrode
NOTE: Silver chloride coating is necessary to allow the electrode reaction: AgCl(s) + e−↔ Ag(s) + Cl–. - Sand a silver wire (thickness ~ 1 mm, length ~ 10 cm) with a fine grade emery paper in order to clean its surface.
- Rinse the silver wire with distilled water.
- Immerse the clean silver-wire in a 4-6% sodium hypochlorite solution (NaClO) until it appears dark (approximately 20 min).
- Use thin glass pipettes filled with an electrolyte solution (2.7 M KCl) as the intracellular electrode.
- Pull a glass capillary tube (internal diameter ≈ 1 mm) with a micro-pipette puller to obtain a thin tip with a small opening (0.01-0.1 µm)14.
- Fill the pulled capillary glass with a 2.7 M KCl solution. First fill it by capillarity (immerse the pipette tip into the 2.7 M KCl solution) and then fill the half of the pipette with a fine injection needle. If necessary, tap the electrode pipette to eliminate air bubbles.
- Connect the electrode to its holder. Connect the holder to the amplifier headstage with the amplifier. Position the electrode-holder-headstage with a stable 3-D micromanipulator. Lower the electrode until bath solution covers its tip.
NOTE: The electrode must have a vertical orientation
- Use a chloride-coated silver-wire electrode as the reference electrode
- Select the Bridge Mode of the amplifier (in the Mode section of the amplifier, press the Bridge button) and measure the electrode resistance. Make sure that the resistance is about 50 MΩ11.
NOTE: This electrode size allows a good-quality electrical recording with limited damage to the cell. - Null the offset current and compensate capacitive transients using the Holding Position button in the section Voltage Clamp of the amplifier, and the Capacitance Neutralization button in Microelectrode 1 section of the amplifier.
- Superfusion system
- Pour the bath solution (step 1.2.2) into a suitable receptacle (a 250 mL serum bottle) and connect it to an irrigation tubing set (3.2 mm of internal diameter), thus connecting the recording chamber. Use a gravity driven superfusion system. Regulate the flow rate to ~ 0.5 mL/s.
- Connect the chamber to a suction device. Regulate the solution suction system in such a way that the total volume of the recording chamber does not vary. Use a vacuum pump for suction.
NOTE: A constant volume in the recording chamber is important to keep stray capacitances constant throughout the experiment.
2. Biological Material
Note: Use adult crayfishes P. clarkii (7-10 cm long) in the intermolt stage of indistinct sex.
- Circadian time
- One month prior to the experiments, maintain 100 crayfishes under a 12-h light/12-h dark cycle15 (white light, 2.4 kW/m2).
NOTE: One month is sufficient time to synchronize the crayfish population. - Determine the circadian time of the crayfish population by assessing the amplitude of electroretinograms of five randomly chosen animals15.
NOTE: 0 h circadian time (CT 0) indicates the beginning of a subjective day, i.e., time during which an organism is normally active16,17,18,19 (Figure 3). The crayfish is a nocturnal animal, so under a 12-h light/12-h dark cycle, it is active during the dark phase.
- One month prior to the experiments, maintain 100 crayfishes under a 12-h light/12-h dark cycle15 (white light, 2.4 kW/m2).
- Eyestalk isolation procedure
- At the desired circadian time, anesthetize a selected crayfish by immersion in tap water at 0-4 °C, for 15 min.
- By means of a fine scissor, detach the eyestalk from the base.
- Access the retina using a razor blade to make an opening (~ 1-mm2) in the dorsal cornea.
- Place the eyestalk in the center of the recording chamber (the hole covered with silicone) with the access opening to the retina on the top of the chamber.
- Keep the eyestalk under constant darkness for 20 min.
NOTE: 20 min is sufficient time for the photoreceptor cell to be fully dark adapted.
3. Photoreceptor Impaling
- Put the microelectrode and the eyestalk's longitudinal axis parallel in such a way that the microelectrode is centered for access to the retina. Use a stereoscopic microscope (10X) to place the devices in the correct configuration.
- Monitor the voltage difference between the reference and recording electrodes by selecting the Bridge Mode11 of the amplifier.
- Lower the electrode into the bath and thereafter place it right over the retina.
- Move away the microscope and position the photostimulator lamp parallel to the eyestalk's longitudinal axis.
NOTE: Distance between lamp and superfusion chamber is approximately 15 cm. - Slowly lower the microelectrode until a sudden voltage drop is detected.
NOTE: A voltage drop of around −50 mV indicates the impalement of a healthy photoreceptor cell. - Deliver a test light-flash (white light, 7.2 kW/m2, 10 µs duration) to record the photoreceptor's receptor potential.
NOTE: The photoreceptor's receptor potential is a depolarizing potential of 10-15 mV amplitude and 300 ms duration (Figure 4).
4. Electrical Recording
- Ensure that the photoreceptor cells are totally adapted to dark conditions (see step 2.3).
- Deliver a test-light flash every 2 min. Automate the time between flashes by choosing an adequate value (120 s) of "time between episodes" in the data acquisition software.
NOTE: 2 min is the necessary time to allow the total recovery of the currents underlying the photoreceptor's electrical response. - Monitor the resting membrane potential, as well as the amplitude and duration of the receptor potential. Assume that the photoreceptor is completely adapted once the membrane potential, amplitude, and duration of the receptor potential remain unchanged. Eyestalks previously kept under constant darkness for 20 min (step 2.3) complete their adaptation in approximately 5 min.
- Stop stimulating the cell 2 min before recording the light-elicited current.
- Deliver a test-light flash every 2 min. Automate the time between flashes by choosing an adequate value (120 s) of "time between episodes" in the data acquisition software.
- Current recording
- Clamp the voltage at the measured resting membrane potential value of the cell by selecting "Holding amplitude" in the data acquisition software (in Waveform on the Analog Output Channel section).
- Select the dSEVC mode of the amplifier. In the Mode section of the amplifier, select the SEVC button and switch the lever to the Discont SEVC position.
- Set the switching rate to 500-1,000 Hz (by using the Rate Adjust button of the amplifier), as determined by the speed of the electrode11.
- Deliver a light-flash, and observe the evoked ion influx.
NOTE: This is the light-elicited or transduction current (Figure 5). - Return to the Bridge mode on the amplifier and record a receptor potential by sending a light flash (see section 3). Make sure that the photoreceptor cell is totally adapted to dark conditions. Measure the receptor potential characteristics (amplitude and duration) and compare with the first measurements. Assume that the impaled photoreceptor cell is a healthy cell if they have the same characteristics.
NOTE: After eyestalk ablation, biological preparation is viable during the following 2 h.
- Two pulse protocol: Measure the recovery from desensitization with a two light-flash protocol.
NOTE: The two light-flash protocol is similar to the standard two-pulse voltage protocol used to measure recovery from inactivation of voltage-gated channels20. The first light-flash causes a temporary change in the sensitivity of the photoreceptor cell and the second flash evaluates the state of the light-activated conductance.- Deliver a pair of light pulses. Apply the second flash after a desired time interval (from 300 ms to 2 min).
- Digitize the currents at 10 KHz sampling with the data acquisition software and save the data for off-line analysis11.
NOTE: A table with the configuration values of the software is included as supplementary material.
5. Data Analysis
- Kinetics of the light-elicited current
- Measure three current parameters: activation latency L, the time elapsed from the light-flash delivery until the current attains 10% of its maximal amplitude (Figure 5); peak or maximal current amplitude Ip; and desensitization time constant T. Measure the desensitization time constant (Τ) by fitting the current decay phase to:

where, A = -I(t=0) is a positive constant (Figure 5).
- Measure three current parameters: activation latency L, the time elapsed from the light-flash delivery until the current attains 10% of its maximal amplitude (Figure 5); peak or maximal current amplitude Ip; and desensitization time constant T. Measure the desensitization time constant (Τ) by fitting the current decay phase to:
- Desensitization and recovery
Note: Recovery from desensitization was assessed as the ratio p2/p1, where p1 is the relevant parameter (either L, Ip, or T) of the control current, and p2 is the corresponding parameter of the second test-current.- Plot p2/p1 (either L, Ip, or T) as a function of time between pulses.
- Depending on the parameter, fit the points of each plot to:
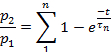
or to:
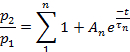
- Use an appropriate statistical test to determine the number of exponential terms required to fit the experimental data.
Representative Results
First, a representative receptor potential of crayfish photoreceptor cells is obtained (Figure 4). Afterwards, a test light-flash was applied to trigger the light transduction current (Figure 5). The cationic transduction current1 activates after a lag, reaching a maximal and thereafter slowly drops into an absorbing desensitized state from which it slowly recovers.
It is reasonable to suppose that the long latency L (tens of milliseconds) of the light-elicited current depends on the biochemical events triggered by light, which involve G-protein pathways. The amplitude of the peak current Ip depends on the fraction of channels available to be opened, and the relative rates of current activation and inactivation. The latter is assessed by the decay time constant T.
On the other hand, the L recovery should be related to the rate of recovery of the biochemical phototransduction cascade; Ip recovery depends both, on the intrinsic conformational changes of the proteins responsible for the ion conductance, and on the rate of recovery of the biochemical phototransduction cascade. The latter could also be related to the recovery of T. Furthermore, the parallel variation of L and T suggest that a common biochemical factor (or factors; e.g., the phosphorylation state of the channel) can affect both parameters1.
A two-flash protocol (Figure 6) was applied subsequently to determine the kinetics of recovery from desensitization (Figure 7) at different moments in the circadian cycle (Figure 8).
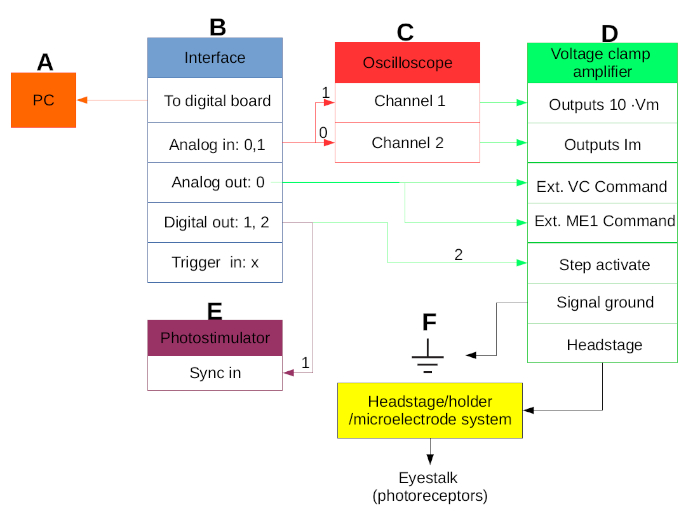
Figure 1. Line diagram of the equipment setup. Connections among personal computer (PC) (A), interface (B), oscilloscope (C), voltage-clamp amplifier (D), photostimulator (E), and the headstage/holder/microelectrode system of the equipment setup (F). Please click here to view a larger version of this figure.
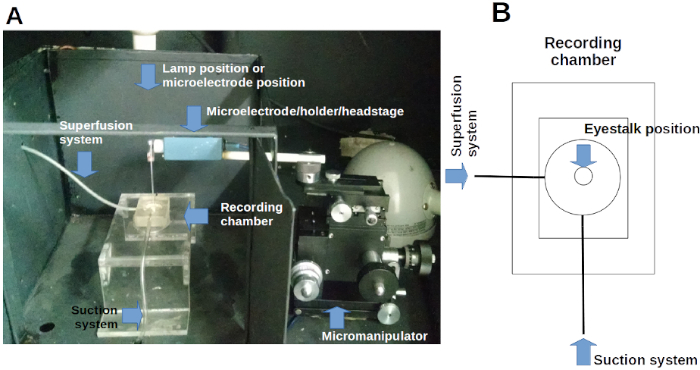
Figure 2. Recording chamber. (A) The recording chamber inside a Faraday cage with its superfusion-suction system. It is also shown the position of photostimulator, microscope, and micromanipulator-headstage-microelectrode system. (B) Diagram of the recording chamber. Please click here to view a larger version of this figure.
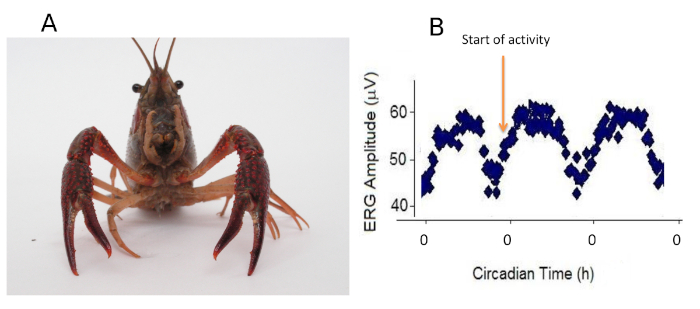
Figure 3. Electroretinogram (ERG) amplitude. (A) Crayfish. (B) Representative plot of the ERG amplitude of crayfish photoreceptors (data were taken every 20 min) as a function of circadian time. The existence of a circadian rhythm can be clearly appreciated. Note that for each cycle, the beginning of activity coincides with circadian time 0. Please click here to view a larger version of this figure.
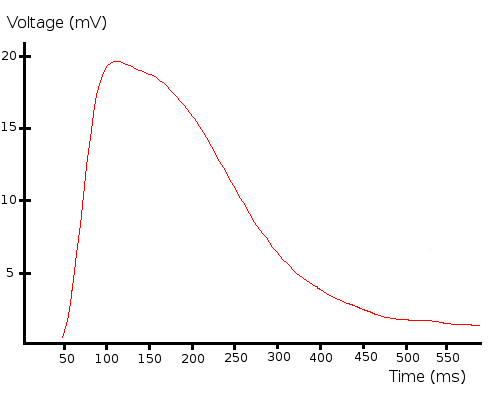
Figure 4. Receptor potential. Representative receptor potential evoked by a light flash (white light, 7.2 kW/m2, 10 µs duration). This figure has been modified from Barriga-Montoya, C, et al.2. Please click here to view a larger version of this figure.
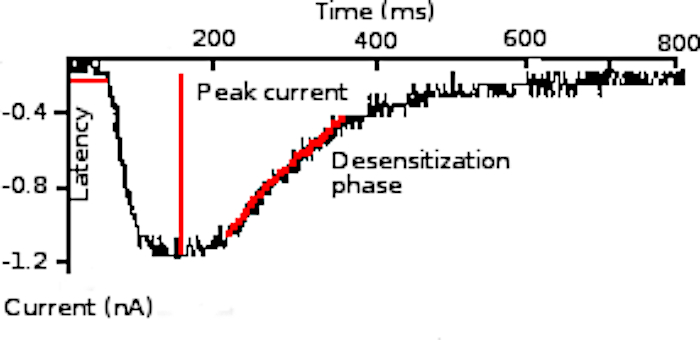
Figure 5. Transduction current. Representative transduction current triggered by a light flash (white light, 7.2 kW/m2, 10 µs duration) applied with the voltage kept constant at the photoreceptor resting potential. Current latency, peak amplitude, and desensitization phase are indicated. This figure has been modified from Barriga-Montoya, C, et al.2. Please click here to view a larger version of this figure.
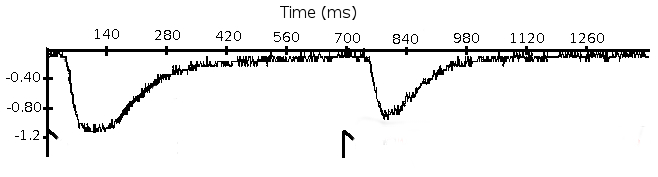
Figure 6. Two-pulse protocol. Currents elicited by a pair of light flashes. Light stimuli were applied at 0 ms and 700 ms (indicated by arrows). This figure has been modified from 2. Please click here to view a larger version of this figure.
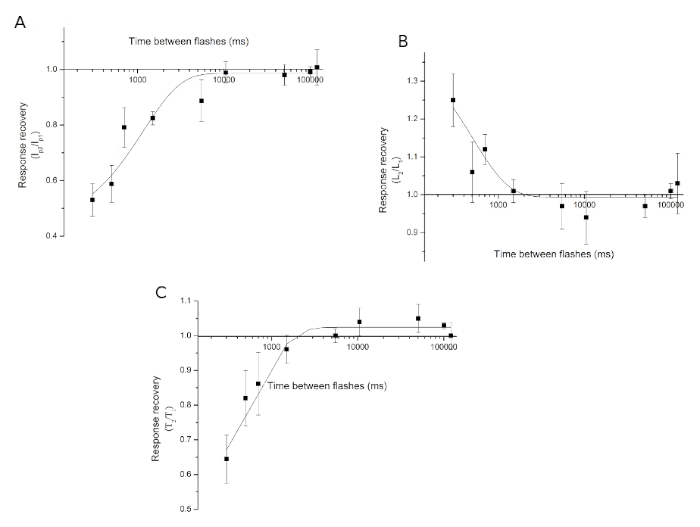
Figure 7. Recovery from desensitization. (A) Ip: peak current recovery, (B) L: latency recovery, (C) T: Desensitization time constant T recovery. Experiments were performed at 0 h CT. This figure has been modified from Barriga-Montoya, C, et al.2. Results are expressed as the mean ± standard deviation of the number of experiments (n = 11). Please click here to view a larger version of this figure.
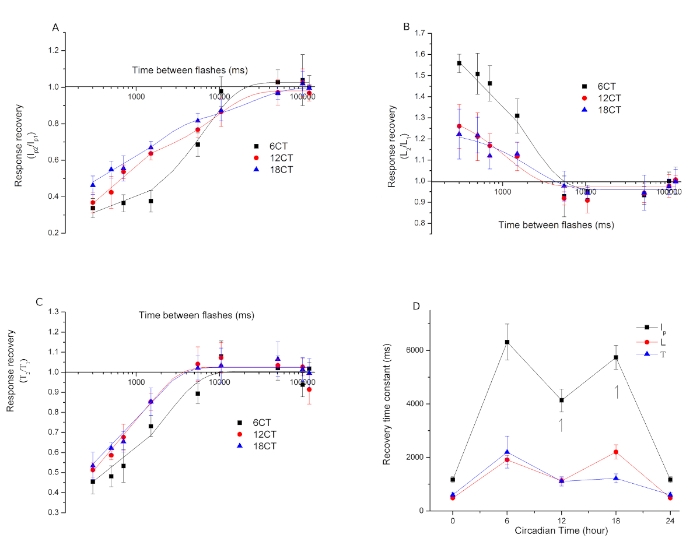
Figure 8. Recovery from desensitization as a function of CT. (A) Ip recovery, (B) L recovery, (C) Т recovery. (D) Weighted time constants. Biphasic processes are marked with an arrow. This figure has been modified from Barriga-Montoya, C, et al.2. Results are expressed as the mean ± standard deviation of the number of experiments (n = 11). Please click here to view a larger version of this figure.
Supplemental Table 1. Please click here to view this table (Right click to download).
Discussion
The crayfish has proven to be an excellent model due to its ability to survive under non-natural conditions. There is easy access to in vivo and in vitro electrophysiological analyses. In addition, crustaceans are a favorable group for neurobiological research in the field of comparative chronobiology21.
In this paper, the study of desensitization and recovery of the light-activated transduction-current of crayfish photoreceptor cells is shown using the intracellular recording technique. However, we believe that the same technique could be adapted to other invertebrate visual systems as long as it is possible to access photoreceptor cells.
To obtain acceptable experimental results, it is important to consider some critical steps in the protocol, including eliminating electrical noise by grounding all equipment, building an electrode with a sufficiently fine tip, and ensuring that the photoreceptor cell is completely dark-adapted before the two-pulse protocol begins.
Despite the limitations of intracellular recording such as the damage of membrane, it is possible to go further and obtain detailed information about the biophysical mechanism underlying crayfish (or another animal) photoreceptor electrical signal. As in other invertebrate photoreceptors, in crayfish photoreceptors, light triggers a graded depolarization, or receptor potential, produced by the activation of a cationic conductance. The light-activated conductance begins after a lag or latency, and after reaching its maximal amplitude, it slowly drops following an exponential time course, as the channels enter an absorbing desensitized state.
The kinetics of recovery from desensitization of the light-elicited ion conductance of crayfish were obtained using a two-light flash protocol (similar to the standard two-voltage pulse protocol used to study recovery from inactivation of voltage-gated channels)20. Interestingly, and in contrast to the well-known case of voltage-gated channels, not only the peak amplitude, but also all the current parameters (Ip, L, T) change after the first light stimulus, recovering with characteristic exponential time courses to the original, first flash values, as reported elsewhere20. This variation of all the parameters that characterize the current indicates the participation of second messengers in the visual transduction system of crayfish1,2,22,23,24.
Additionally, and interestingly, as Figure 8 shows, recovery of L, Ip, and T depends on the circadian time at which experiments are realized. Further studies are needed to determine the molecular basis of this phenomenon. Of course, it is possible to obtain similar information using another technique, such as patch clamp. However, the intracellular milieu can be greatly disturbed in other techniques, and this could be critical, if, for example, internal elements like proteins, amino acids, nucleotides, among many others, play a relevant role in the electrical signal generation or in the interaction with hormones or neuromodulators.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This work was supported by DGAPA-UNAM IN224616-RN224616 grant. The authors want to thank Mrs. Josefina Bolado, Head of the Scientific Paper Translation Department, from División de Investigación at Facultad de Medicina, UNAM, for editing the English-language version of this manuscript.
Materials
| Axoclamp2A | Axon Instruments Inc | Amplifier | |
| Digidata 1200 Interface | Axon Instruments Inc | Digitizer | |
| Oscilloscope TDS430A | Tektronix | Analogic Oscilloscope | |
| Photostimulator PS33 Plus | Grass | Lamp | |
| Puller PC-100 | Narishige | Micropipette Puller | |
| Puller P-97 | Sutter Instruments | Micropipette Puller | |
| Glass Capillary Tube Kimax-51 | Kimble Products | 34502 | 0.8, 1.10, 100 mm |
| HS-2 Headstage | Axon Instruments Inc | Headstage | |
| Micromanipulator MX-4 | Narishige | Mechanical Micromanipulator | |
| Stereoscopic Microscope | Zeiss | Microscope | |
| pClamp | Axon Instruments Inc | Data acquisition software for digidata 1200 interface | |
| Clampfit | Axon Instruments Inc | Analysis software linked to pClamp | |
| Origin | OriginLab Corp. | Data analysis and graphing software | |
| Sodium Chloride | Sigma | S7653 | >99.5% |
| Potassium Chloride | Sigma | P-9333 | Minimum 99% |
| Magnesium Sulfate | Sigma | M7506 | Minimum 99.5% |
| Calcium Chloride | Sigma | C5080 | Minimum 99.0% |
| Hepes | Sigma | H7523 | >99.5% |
| Sodium Hydroxide | Sigma | S8045 | 98.00% |
| Sodium hypochlorite solution | Sigma | 425044 | Available chlorine, 10-15% |
Referanslar
- Barriga-Montoya, C., Gómez-Lagunas, F., Fuentes-Pardo, B. Effect of pigment dispersing hormone on the electrical activity of crayfish visual photoreceptors during the 24-h cycle. Comp. Biochem. Physiol. A Comp. Physiol. 157 (4), 338-345 (2010).
- Barriga-Montoya, C., de la O-Martínez, A., Fuentes-Pardo, B., Gómez-Lagunas, F. Desensitization and recovery of crayfish photoreceptors. Dependency on circadian time, and pigment-dispersing hormone. Comp. Biochem. Physiol. A Comp. Physiol. 203, 297-303 (2017).
- Wickenden, A. D. Overview of electrophysiological techniques. Curr. protoc. pharmacol. 11, 1-17 (2014).
- Brette, R., Destexhe, A. Intracellular recording. Handbook of Neural Activity Measurement. , 44-91 (2012).
- Cummins, D., Goldsmith, T. H. Cellular identification of the violet receptor in the crayfish eye. J. Comp. Physiol. 142 (2), 199-202 (1981).
- Eguchi, E. Rhabdom structure and receptor potentials in single crayfish retinular cells. J. Cell and Comp. Physiol. 66, 411-430 (1965).
- Nosaki, H. Electrophysiological study of color encoding in the compound eye of crayfish, Procambarus clarkii. Z. vergl. Physiologie. 64 (3), 318-323 (1969).
- Miller, C. S., Glantz, R. M. Visual adaptation modulates a potassium conductance in retinular cells of the crayfish. Vis Neurosci. 17 (3), 353-368 (2000).
- Hamill, O. P., Marty, A., Neher, E., Sakmann, B., Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391 (2), 85-100 (1981).
- Lishko, P., Clapham, D. E., Navarro, B., Kirichok, Y. Sperm patch-clamp. Methods Enzymol. 525, 59-79 (2013).
- Finkel, A. Axoclamp-2A microelectrode clamp theory and operation. Axon Instruments, Inc. , (1990).
- Sakmann, B. . Single-channel recording. , (2013).
- Van Harreveld, A. A physiological solution for freshwater crustacea. Proc. Soc. Exp. Biol. Med. 34 (4), 428-432 (1936).
- Oesterle, A. . pipette cookbook. , 97 (2008).
- Fuentes-Pardo, B., Ramos-Carvajal, J. The phase response curve of electroretinographic circadian rhythm of crayfish. Comp. Biochem. Physiol. A Comp. Physiol. 74 (3), 711-714 (1983).
- Pace-Schott, E. F., Hobson, J. A. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 3 (8), 591-605 (2002).
- Pittendrigh, C. S., Aschoff, J. On the mechanism of the entrainment of a circadian rhythm by light cycles. Circadian Clocks. , 277-297 (1965).
- Pittendrigh, C. S., Aschoff, J. Circadian systems: entrainment. Handbook Behavioral Neurobiology Biological Rhythms. , 94-124 (1981).
- Vitaterna, M. H., Takahashi, J. S., Turek, F. W. Overview of circadian rhythms. Alcohol Res. Health. 25 (2), 85-93 (2001).
- Hille, B. . Ion channels of excitable membranes. 507, (2001).
- Dircksen, H., Strauss, J. Circadian clocks in crustaceans: identified neuronal and cellular systems. Front Biosci. 15, 1040-1074 (2010).
- Terakita, A., Hariyama, T., Tsukahara, Y., Katsukura, Y., Tashiro, H. Interaction of GTP-binding protein Gq with photoactivated rhodopsin in the photoreceptor membranes of crayfish. FEBS Lett. 330, 197-200 (1993).
- Terakita, A., Takahama, H., Hariyama, T., Suzuki, T., Tsukahara, Y. Light regulated localization of the beta-subunit of Gq-type G-protein in the crayfish photoreceptors. J Comp Physiol A. 183 (4), 411-417 (1998).
- Terakita, A., Takahama, H., Tamotsu, S., Suzuki, T., Hariyama, T., Tsukahara, Y. Light-modulated subcellular localization of the alpha-subunit of GTP-binding protein Gq in crayfish photoreceptors. Vis Neurosci. 13 (3), 539-547 (1996).

