A Device for Performing Cell Migration/Wound Healing in a 96-Well Plate
Özet
Here, we present a protocol to perform a semi-high throughput cell migration assay on a 96-well cell culture plate. This protocol is a fast, simple and economical method to create consistent scratch wounds on a cell monolayer.
Abstract
The cell migration/wounding assay is a commonly used method to study cell migration and other biological processes, such as angiogenesis and tumor metastasis. In this assay, cells are grown to form a confluent monolayer and a mechanical wound is created by scratching with a device. Then the migration rate of the cells towards the denuded area can be monitored by imaging. Our 8-channel mechanical wounder is designed to tackle most of the problems associated with the cell migration assay. Firstly, our wounder can be easily sterilized by autoclaving or with common disinfectants. Secondly, the individual adjustable pins allow even contact with the cell culture plate so that sharp and reproducible wounds can be created. Thirdly, the guiding bars on both sides of the wounder ensure consistent wounding position in each well. The use of disposable plastic pipette tips for wounding can further provide better handling of the wounder as well as to minimize cross-contamination. In conclusion, our cell wounder can provide researchers with a user friendly and reproducible device for performing the cell migration assay using the standard 96-well culture plate.
Introduction
Cell migration plays an important role in cellular processes, such as embryogenesis, neurogenesis, angiogenesis, wound healing, mucosal repairing, epithelial-mesenchymal-transitions under normal development and disease situation1. It is a complicated process which involves the coordination of numerous inter- and intra-cellular events including signaling molecule interactions, cell polarization, cytoskeletal reorganization, matrix remodeling, membrane protrusion and dynamic cell-cell adhesion modulation2. By studying cell migration, the discovery and validation of chemicals or biomolecules leading to cell movement and its related biochemical pathways can be determined. This fundamental process can be used beneficially as a proxy for various applications, such as disease treatment and drug targeting.
The wounding assay is one of the cell migration assays3. Here, an individual sample of cells will be partially removed by mechanical means to produce a denuded area where cells will migrate to cover the area. The percentage of recovery at a particular time point will be monitored. When handling large number of samples, issues such as the experimental cost and cross-contamination within samples can be problematic. Although many commercial tools and assays are available for studying cell migration4,5,6, many of them required expensive and sophisticated equipment and improvements are still needed. For these reasons, an 8-channel mechanical cell wounder (Figure 1) is developed.
Our 8-channel mechanical cell wounder has incorporated several unique features in solving the above-mentioned problems. It provides the flexibility to perform cell migration/wounding assays in the 96-well culture plate format. The adjustable guiding bar design ensures that the scratch area is in the central position of each well. Also, the height of the guiding bars can be adjusted so that the wounder is applicable for various brands of culture plates. Finally, the adjustable wounding pin design enables an even contact of the pipette tips with the culture plate surface to achieve simultaneous and reproducible wounding7,8.
Protocol
1. Introduction to the Parts of the Wounder (Figure 1)
- Prepare the pin holder by fixing the wounding pins at equal distances. Hold the pipette tips and adjust the tip height by moving the adjustable wounding pins up and down.
- Fit the adjustable guiding-bar on different brands of 96-well culture plates and ensure that the wounding area is in a fixed position.
- Fix the adjustable wounding pin by tightening the hex screw. Fix the guiding-bar in position by tightening the hex socket head caps on both ends.
2. Setting the Width of the Pin Holder (Figure 2)
- Loosen the hex socket head caps with the M5 hex wrench.
- Insert a suitable number of adjusting rings to both sides of the pin holder so that the guiding bars fit perfectly with the width of the 96-well culture plate.
NOTE: Corning and Iwaki plates requires 1 pair of large rings and 1 pair of small rings. Falcon and Nunc plates need 1 pair of large rings. - Tighten the hex socket head caps to fix the guiding bar.
3. Adjusting the Guiding Bars (Figure 3)
- Fit the wounding pins with sterile 10-µL plastic pipette tips.
- Loosen the hex socket head caps with the M5 hex wrench.
- Hold the wounder and dip the wounding pins into one column of a 96-well culture plate.
- Adjust the height of the guiding bars until all of the tips barely touch the bottom of the wells.
- Tighten the hex socket head caps. Ensure that the wounder now perfectly fits on the culture plate and that the pins are well-positioned in the middle of the wells.
4. Calibration of the Pins
- Hold the wounder perpendicular to a flat sterile surface (i.e. Petri dish) and loosen all the hex screws with the M3 hex wrench.
- Tap the wounder until all of the tips evenly touch the flat sterile surface.
- Tighten the hex screws again to lock the pins in position.
- Check the evenness of each tip by tapping the wounder gently on the flat sterile surface.
5. Scratching Cell Monolayer (Figure 4)
- Seed human umbilical vascular endothelial cells on a 0.1% gelatin-coated 96-well cell culture plate. Culture cells in Medium 199 supplemented with 20% heat-inactivated fetal bovine serum, 1% penicillin/streptomycin and 0.09 g/L heparin. Maintain cells in a humidified incubator with 5% carbon dioxide at 37 ºC overnight before being used. Cells should reach 100% confluency before wounding.
- Place the wounding tips at the leftmost (or the rightmost) side of each well within the same column of the culture plate. Slide the wounder across to the other side of the well; make sure the wounding tips are touching the bottom of the well.
- Repeat the scratching (Step 5.2) for all columns.
- After wounding, discard the medium in each well and replace with fresh medium containing testing compounds.
6. Data Acquisition and Image Analysis
- Image the whole well with the mechanical wound on the cell monolayer using a low-power microscope with digital camera (e.g. 10X objective) immediately after wounding (t=0 h).
- Add test compounds if desired and incubate for the desired length of time. Image the whole well with the wound area again after the desired incubation time (t=Δh).
- Using image analysis software such as ImageJ (https://imagej.nih.gov/ij/), measure the wounded area on the image using the freehand tool.
- Calculate the percentage of wound closure:
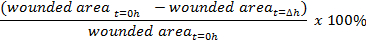
Representative Results
This 8-channel mechanical wounder is constructed to scratch a cell monolayer in order to perform the cell migration assay. It is a user-friendly device that can carry out cell scratching assays in 96-well plates in less than a minute without special training. This wounder can introduce wound areas on cell monolayers with a uniform width of about 600 µm and with sharp edges (Figures 5 and 6). The width of the wounds depends on the brand of pipette tips and the user's performance. This range of wound width is suitable for common image capturing under low-power magnification (Figure 7). After capturing the image, the percentage of wound closure can be calculated. This cell wounder has incorporated different strategies in solving problems encountered with the cell migration assay. Also, it provides flexibility in performing cell migration assays in various 96-well culture plate formats.
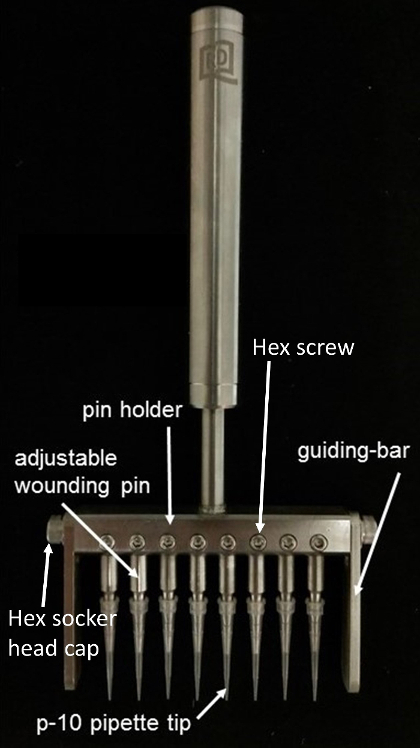
Figure 1: Eight-channel mechanical cell wounder. Please click here to view a larger version of this figure.
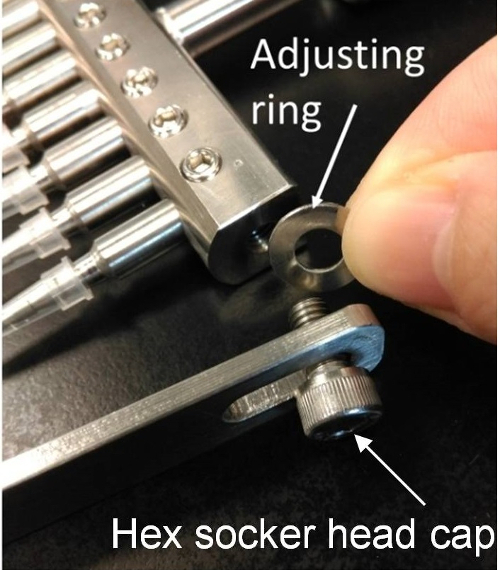
Figure 2: Adjust the width of the pin holder. Insert the adjusting rings in order to fit the 96-wells plate. Please click here to view a larger version of this figure.
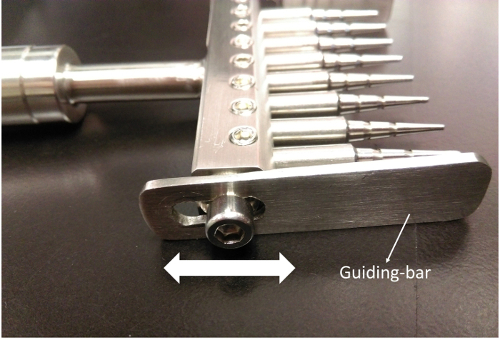
Figure 3. Adjust the guiding bars to fit different plate formats. Slide the guiding bar up and down to fit the 96-wells plate. Please click here to view a larger version of this figure.
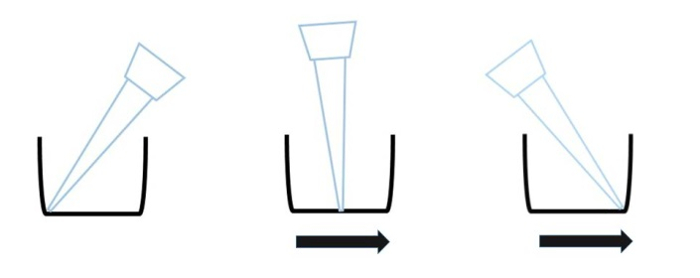
Figure 4: Scratching direction of the tip. Scratch the cells by sliding the wounder from the one side of the well bottom to the other side of the well bottom.
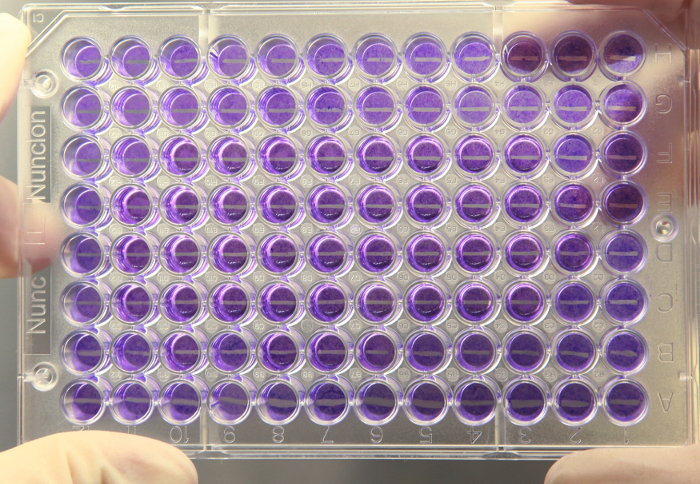
Figure 5: Overview of the 96-well plate after wounding. The cell monolayer is wounded by the wounder producing sharp edges with uniform width of about 600 µm. Please click here to view a larger version of this figure.
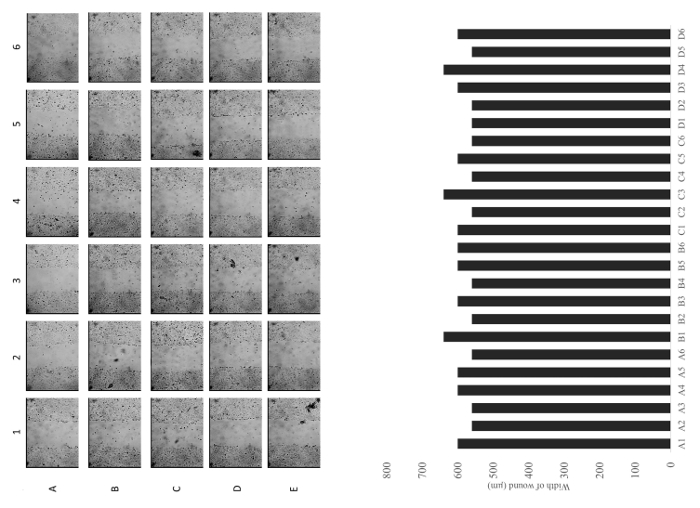
Figure 6: Representative images of a row and a column of wells immediately after scratching. Quantitative analysis of the width of the each wound. Images are captured under a 10X objective. Please click here to view a larger version of this figure.
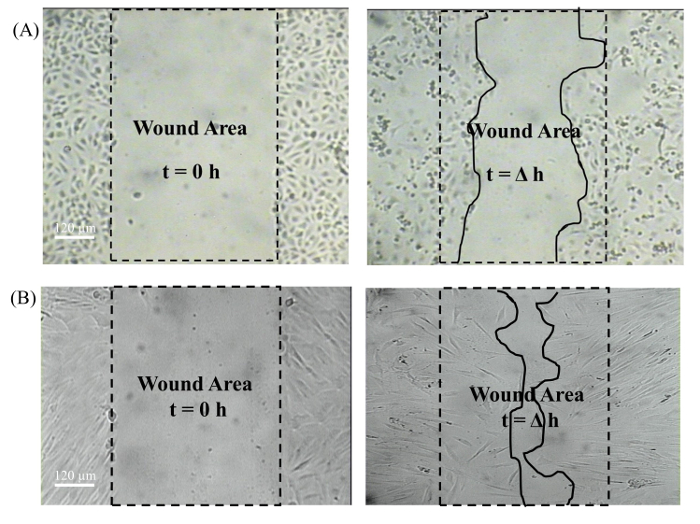
Figure 7: Captured wound images (10X objective) at t=0 h and t=Δh. The dotted line indicates the wound area and the solid line indicates the cell migration area. A) Human umbilical vein endothelial cells. B) Human dermal fibroblast. Please click here to view a larger version of this figure.
Discussion
Our cell wounder has several unique features in solving the problems of traditional cell migration assays. The 8-channel mechanical cell wounder is made of high grade stainless steel (Steel 304) with a long lifespan that can be sterilized by autoclaving. Almost all commercial brands of 96-well culture plates available on the market can be used with this mechanical cell wounder because of the adjustable guiding bar. The design of the adjustable guiding bar also ensures that the scratching area is at the center of each well. The wound in a central position of the well can facilitate image capturing using a microscope. The images possess some variations while taken at different time points, but they do not affect data analysis because almost 90% of the well area can be viewed by the microscope with a low-magnification objective (i.e. 10X). Thus, a semi-high throughput analysis can be performed on 96-well culture plates7,8. However, this method still requires a large number of images, which require lengthy data analysis. This may be solved if an automatic image acquiring system is available.
The design of the individual adjustable pins of the wounder allows even contact with the 96-well plate surface to achieve identical, smooth and sharp wounds on the cell monolayer. The scratching pins are commercial plastic pipette tips, which can be sterilized and disposed after every single scratching, and can provide great elasticity to contact the well surface without mechanical damage. These advantages can further minimize cross contamination of samples during the experiment and the influence on cell movement across the denuded area. In the future, we will develop a spring-controlled pin which can eliminate the pin height adjustment step. The most critical step to use this device is the force applied on the cell monolayer during the scratching. The wounder must be held perpendicular against the culture plate, so that eight pipette tips are touching the bottom of the well. Indeed, the wounder provides a method to perform the cell migration assay without the use of expensive and complicated equipment. The protocol for this wounder is simple and straightforward.
In conclusion, our 8-channel mechanical wounder is a user-friendly device for cell migration assays that requires no special skills. Users can perform the cell migration assay in 96-well plates with a relatively large number of samples in a fast and simple manner9,10,11.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Mr. Tam Po Leung, Mr. Wong Chi Kin and the technical staff of the Science Faculty Workshop, Hong Kong Baptist University, for their technical skills and advice in making the prototype of this wounder.
Materials
| 96-well cell culture plate | Nunc | 167008 | Other brands of 96-well cell culture plate can also be used |
| P10 pipette tips | Axygen | 301-03-051 | Short P10 pipette tip is more easy to create a clear wound |
| Wounder | R&P Technology Limited | ||
| Medium 199 | Sigma | M2520-1L | For cell culture of human umbilical vein endothelial cells. Use appropirate culture medium and condition for other type of cells. |
| Fetal bovine serum | Gibco | 26140079 | For cell culture of human umbilical vein endothelial cells. Use appropirate culture medium and condition for other type of cells. |
| Penicillin/Streptomycin | Gibco | 15140122 | For cell culture of human umbilical vein endothelial cells. Use appropirate culture medium and condition for other type of cells. |
| Heparin sodium salt from porcine intestinal mucosa | Sigma | H3393 | For cell culture of human umbilical vein endothelial cells. Use appropirate culture medium and condition for other type of cells. |
| Gelatin from bovine skin | Sigma | G9391 | For cell culture of human umbilical vein endothelial cells. Use appropirate culture medium and condition for other type of cells. |
Referanslar
- Friedl, P., Wolf, K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 3 (5), 362-374 (2003).
- Friedl, P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 16 (1), 14-23 (2004).
- Lampugnani, M. G. Cell migration into a wounded area in vitro. Methods Mol Biol. 96, 177-182 (1999).
- Sholley, M. M., Gimbrone, M. A., Cotran, R. S. Cellular migration and replication in endothelial regeneration: a study using irradiated endothelial cultures. Lab Invest. 36 (1), 18-25 (1977).
- Gotlieb, A. I., Spector, W. Migration into an in vitro experimental wound: a comparison of porcine aortic endothelial and smooth muscle cells and the effect of culture irradiation. Am J Pathol. 103 (2), 271-282 (1981).
- Chen, Y. C., et al. Single-cell migration chip for chemotaxis-based microfluidic selection of heterogeneous cell populations. Sci Rep. 18 (5), 9980 (2015).
- Yarrow, J. C., Periman, Z. E., Westwood, N. J., Mitchison, T. J. A high-throughput cell migration assay using scratch wound healing, a comparsion of image-based readout methods. BMC Biotechnol. 4, 21 (2004).
- Lauder, H., Frost, E. E., Hiley, C. R., Fan, T. P. Quantification of the repair process involved in the repair of a cell monolayer using an in vitro model of mechanical injury. Angiogenesis. 2 (1), 67-80 (1998).
- Yue, P. Y. K., Leung, E. P. Y., Mak, N. K., Wong, R. N. S. A simplified method for quantifying cell migration/ wound healing in 96-well plates. J. Biomol Screen. 15 (4), 427-433 (2010).
- Yue, P. Y., et al. Elucidation of the mechanisms underlying the angiogenic effects of ginsenoside Rg(1) in vivo and in vitro. Angiogenesis. 8 (3), 205-216 (2005).
- Kwok, H. H., Chan, L. S., Poon, P. Y., Yue, P. Y., Wong, R. N. Ginsenoside-Rg1 induces angiogenesis by the inverse regulation of MET tyrosine kinase receptor expression through miR-23a. Toxicol Appl Pharmacol. 287 (3), 276-283 (2015).

