A Protocol for the Production of Gliadin-cyanoacrylate Nanoparticles for Hydrophilic Coating
Özet
This article presents a protocol for the production of protein-based nanoparticles that changes the hydrophobic surface to hydrophilic. The produced nanoparticle is an assembly of gliadin-cyanoacrylate diblock copolymers. Spray coating with the produced nanoparticle changes the surface of target material to a hydrophilic surface.
Abstract
This article presents a protocol for the production of protein-based nanoparticles that changes the hydrophobic surface to hydrophilic by a simple spray coating. These nanoparticles are produced by the polymerization reaction of alkyl cyanoacrylate on the surface of cereal protein (gliadin) molecules. Alkyl cyanoacrylate is a monomer that instantly polymerizes at RT when it is applied to the surface of materials. Its polymerization reaction is initiated by the trace amounts of weakly basic or nucleophilic species on the surface, including moisture. Once polymerized, the polymerized alkyl cyanoacrylates show a strong affinity with the object materials because nitrile groups are in the backbone of poly (alkyl cyanoacrylate). Proteins also work as initiator for this polymerization because they contain amine groups that can initiate the polymerization of cyanoacrylate. If aggregated protein is used as an initiator, protein aggregate is surrounded by the hydrophobic poly(alkyl cyanoacrylate) chains after the polymerization reaction of alkyl cyanoacrylate. By controlling the experimental condition, particles in the nanometer range are produced. The produced nanoparticles readily adsorb to the surface of most materials including glass, metals, plastics, wood, leather, and fabrics. When the surface of a material is sprayed with the produced nanoparticle suspension and rinsed with water, the micellar structure of nanoparticle changes its conformation, and the hydrophilic proteins are exposed to the air. As a result, the nanoparticle-coated surface changes to hydrophilic.
Introduction
The goal of this article is to show the protocol for the preparation of nanoparticle suspension that modifies the wetting property of materials by a simple spray. The presented nanoparticle suspension is made from alkyl cyanoacrylate1 and a cereal protein, gliadin2,3. During the manufacturing process, protein aggregates are formed in aqueous ethanol4. Subsequent reaction with monomer (alkyl cyanoacrylate) produces the nanoparticle that is comprised of a protein core surrounded by linear polymer chains [poly(alkyl cyanoacrylate)]5.
Poly(alkyl cyanoacrylate)s are biodegradable and have been used for the production of nanoparticles via emulsion polymerization6. This reaction is spontaneously initiated by the hydroxyl groups dissociated from water or by other nucleophilic groups in the reaction medium7. In the case of the reaction presented in this article, the amine groups on the surface of protein aggregates initiate the polymerization reaction of alkyl cyanoacrylate monomers5,8. As a result of this reaction, nanoparticles are formed in the reaction medium. The core of the nanoparticle is protein aggregates and the outer layer is poly(alkyl cyanoacrylate) (PACA) chains. The prepared nanoparticle has a strong affinity on most materials (more precisely, any material which PACA can adsorb to) and adheres onto their surface to form a thin coating on a nanometer scale. A simple spray coating instantly turns the surface of the materials hydrophilic.
Gliadin is one of the main fractions of gluten, which is in the endosperms of wheat. Gliadins are mainly monomeric proteins with molecular weights around 28,000 – 55,000. Non-covalent bonds such as hydrogen bonds, ionic bonds and hydrophobic bonds are responsible for the aggregation of gliadins2. Although gliadin is chosen as a reactant in this article, many other proteins can also be used for the same purpose. However, the reaction condition needs to be modified accordingly because the condition for inducing aggregation is dependent on the type of protein to be employed8. Compared with other proteins, gliadin is more readily available, purification is simple, and production cost is low. Although ethyl cyanoacrylate (ECA) is chosen as a monomer for the presented reaction, other alkyl cyanoacrylates can also be used for the same reaction. The reason for choosing ECA is that it is readily available at low cost.
Protocol
1. Defatting Commercial Gliadin
- Measure 150 ml of acetone with a graduated cylinder and pour into 250 ml Erlenmeyer flask.
- While stirring with a spin bar on a magnetic stirrer at RT, add 30 g of commercial gliadin powder. Seal the opening of flask with aluminum foil, and keep on stirring O/N in the hood.
- Filtrate the solution with a filter paper.
- Wash the filtrate with fresh acetone (ca. 50 ml). Let stand for 10 min to allow the acetone to drain.
- Transfer the filtrate together with the filter paper underneath to a large dish such as a cell culture square dish. Cover the whole dish with a large filter paper to slow down the evaporation of residual acetone.
- Allow the filtrate to dry completely in the hood O/N. Store the defatted gliadin in an air-tight container at RT.
2. Preparation of Purified Gliadin
- Measure 150 ml of water with a graduated cylinder and pour into 1,000 ml Erlenmeyer flask.
- Measure 350 ml of absolute ethanol with a mess cylinder and pour into the same 1,000 ml Erlenmeyer flask. Stir vigorously (800 – 1,000 rpm) with a spin bar until no bubble is generated from the solution mixture.
- While stirring, add 20 g of defatted-commercial gliadin powder. Add gliadin in small amounts at a time to avoid the formation of lumps that contains air in the middle. Keep on stirring O/N.
- Transfer the whole solution to a 1,000 ml mess cylinder. Let stand for two days. During this time, impurities will be precipitated at the bottom of the cylinder.
- Transfer the supernatant to a rotary evaporator with a pipette, and remove ethanol from the supernatant as much as possible. As the percentage of ethanol is reduced, gliadin will appear in the solution as aggregate.
- Freeze the solution containing gliadin aggregates by immersing/rotating in methanol/dry ice mixture and freeze-dry at -70 °C under vacuum. Before freeze-drying, make sure the whole solution is frozen without any liquid.
NOTE: The freeze-dried gliadin will form a porous solid. - Crush the freeze-dried gliadin with mortar and pestle, and grind with a coffee grinder to obtain a fine power (<0.5 mm). Transfer the product into an air-tight container and store at RT.
3. Polymerization Reaction of ECA with Gliadin
- Place a scintillation vial (volume is around 20 ml) in a weighing balance, and tare. Add 3.2 g distilled water and 6.8 g absolute ethanol in the scitillation vial.
- Move the scintillation vial on to a magnetic stirrer, put a magnetic spin bar (20 x 3 mm) into the vial, and stir vigorously (800 – 1,000 rpm) until no air bubble is generated from the aqueous ethanol mixture.
- Add 40 µl of 4 N HCl into the vial while stirring. Add 20 mg of gliadin into the vial while stirring, and keep on stirring until gliadin powder is fully dissolved in the aqueous ethanol mixture.
- After making sure the gliadin solution is clear to the naked eyes, slowly add 80 – 100 µl of ECA while stirring speed is still 800 – 1,000 rpm.
- Lower the stirring speed to 500 rpm, and continue stirring for 1 hr. As the reaction proceeds, observe turbidity which indicates that the polymerization reaction is in progress.
- When the reaction is terminated, transfer the solution into centrifuge tubes, and balance the weight of the tubes. Centrifuge the reaction product at 10,000 x g for 20 min. During this time, side product precipitates and nanoparticles remain in the supernantant. The major component of side product is PACA homopolymer.
- After the centrifuge, transfer the produced nanoparticle suspension (supernatant) with a pipette to a scintillation vial (or any air-tight container), and store it at RT.
4. Characterization of the Product
- Prepare 10 g of 68 wt% aqueous ethanol by mixing 3.2 g water and 6.8 g absolute ethanol in 20 ml scintillation vial and stir until no bubble is generated from the solution mixture.
- Add 40 µl of 4 N HCl to the solution mixture while stirring. Add 50 µl of nanoparticle suspension to the prepared ethanol solution while stirring.
- Measure the size of nanoparticle with Dynamic Light Scattering (DLS) by using the sample solution prepared above and follow manufacturer's instructions.
NOTE: The size of the final product has to be smaller than 200 nm. If it is larger than 200 nm, the product will not be stable for an extended period of time. Larger nanoparticles are produced when the employed ECA is not fresh.
5. Examination of the Product
- Prepare a glass plate such as a hand mirror. Wash the surface of glass plate with soap water. Rinse the surface of glass plate with flowing water. Dry the cleaned surface or use without drying for the next step.
- Spray the nanoparticle suspension obtained in 3.6) on a part of the glass plate, and rinse off the surface with flowing water immediately.
- Spray pure water on the surface of glass plate. Observe the coated (sprayed with nanoparticle suspension) surface hold water layer while the uncoated surface is clear.
Representative Results
Nanoparticles can be prepared in various reaction conditions. Gliadin forms aggregate in broad range of ethanol content5. However, the size of aggregates needs to be as small as possible because an additional layer (i.e., polymerized ECA) will be added to this aggregate and this process will make the final size larger. If the final size of particle is too large, the particle will be unstable and will easily be precipitated. Therefore, 68% aqueous ethanol was chosen as a reaction medium4. The size of the produced nanoparticle is on the order of a few hundred nanometers. The working mechanism for the hydrophilic coating with the produced nanoparticle is as follows.
For the coating of materials, this nanoparticle suspension (suspended in 68% ethanol) is sprayed on the surface of target material followed by rinsing off with water. When the nanoparticles suspended in aqueous ethanol is sprayed on the surface of target material, aqueous ethanol easily spreads to a broader area because the surface tension of ethanol is very low. This spreading action of aqueous ethanol delivers the suspended nanoparticles on the surface of the target material quickly and evenly. The delivered nanoparticles adsorb onto the material because of the strong affinity of PECA chains on the surface of nanoparticles. Subsequent addition of water changes the conformation of nanoparticles. In other words, the nanoparticle opens its structure to expose the inner hydrophilic proteins to the air. As a result of this conformational change, the surface of the coated material turns hydrophilic8. To understand this conformational change of nanoparticles, this situation is mimicked by putting the nanoparticles into various ethanol solutions and monitoring the zeta potential.
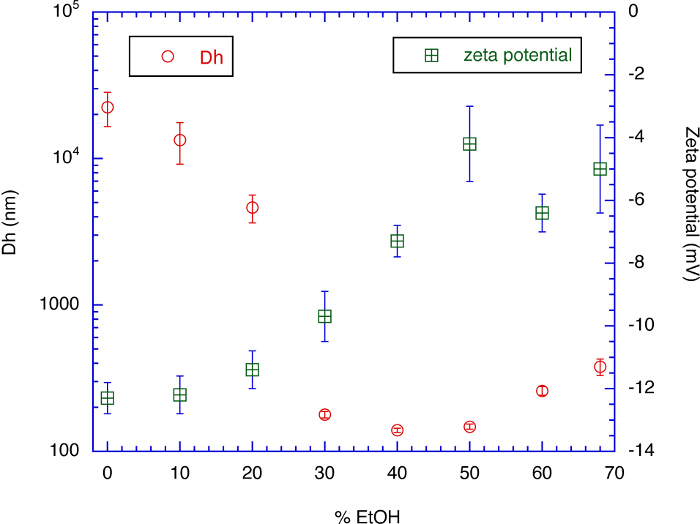
Figure 1. Zeta Potential and Diameter of the Produced Nanoparticles in Various Aqueous Ethanol Solutions. This graph shows that each nanoparticle changes its conformation and its surface becomes hydrophilic as the ethanol content of suspension medium decreases. This is the working mechanism of the hydrophilic coating with nanoparticles. Error bars represent standard error of the mean. Please click here to view a larger version of this figure.
Figure 1 shows that the nanoparticle prepared in 68% aqueous ethanol is disintegrated into smaller particles as the surrounding suspension medium contains less ethanol, from 68% to 50%. In this ethanol content range, error bars of zeta potential are large, meaning that the distribution of surface charge is quite broad and particles are not well stabilized. As the content of water increases, the absolute value of zeta potential rapidly increases until the ethanol content decreases to 30%. This means that the surface of each nanoparticle is highly charged and the hydrophilicity of particle surface is significantly increased. After that, the zeta potential does not change much because nanoparticles form aggregates. This aggregate formation does not happen when the nanoparticle suspension is used for hydrophilic coating because the particles are adsorbed on the surface of materials. In short, the process for the hydrophilic coating involves spraying the nanoparticle suspension, adsorption of nanoparticles on the surface of target material, and exposure of inner protein molecules to the air. In reality, the whole coating process takes place in less than a minute.
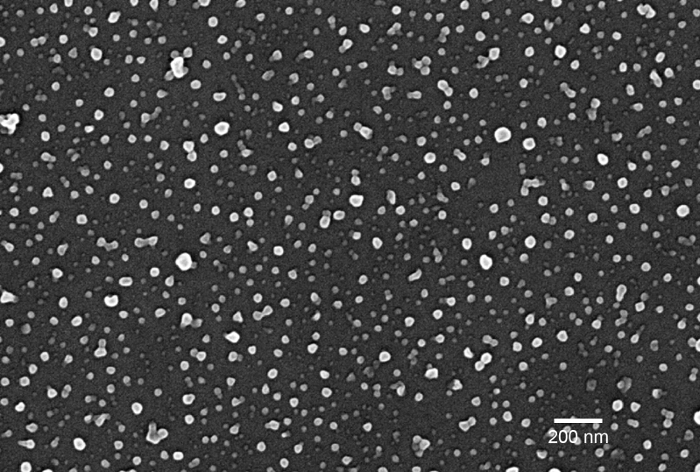
Figure 2. SEM Image of Adsorbed Nanoparticles. SEM shows the distribution of nanoparticles on the surface of a glass plate. Because of the evenly distributed nanoparticles, the transmittance of visible light is not significantly affected. Please click here to view a larger version of this figure.
As a result of the coating with nanoparticles, particles are adsorbed on the surface of the target material. Figure 2 shows the nanoparticles adsorbed on the surface of a glass plate. This image was taken with a Field Emission-Scanning Electron Microscope (SEM). This SEM image shows that all the particle sizes look smaller than 100 nm. Since the sample was sputter coated prior to SEM imaging, however, there should be some shrinkage of particle size during this process. This SEM image shows that nanoparticles are well dispersed on the surface of a glass plate and there are enough spaces between particles for the light to pass through. This spatial distribution of particles and the small size of particles (i.e., smaller size than the wavelength of visible light) explain why the transparency of the glass plate is not much affected by the coating.
| Before coating | After coating | |
| Glass | 25.5 ± 1.5° | 8.9 ± 0.8° |
| Polystyrene | 52.7 ± 1.2° | 6.8 ± 0.8° |
Table 1: Contact Angle of Substrates Before & After Coating with Nanoparticles.
The produced nanoparticle adsorbs on the surface of various materials. Once the target material is coated with nanoparticles, the contact angle of the coated surface drops significantly. Table 1 shows the experimental data measured by a dynamic contact angle (DCA) instrument. In this test, the Wilhelmy plate method was used to determine the effect of nanoparticle coating on surface wettability9. For the measurement of contact angle, samples were prepared by dipping plates made of the material to be tested into the nanoparticle suspension and rinsing with a stream of distilled water for a few seconds. The prepared plate was consecutively immersed in and removed from distilled water. Curves relating the interfacial tension to the immersion depth were plotted and used to calculate the receding contact angle10. In the table, it is shown that the surface tension of two examples, glass and a plastic (polystyrene), drops significantly after the coating.
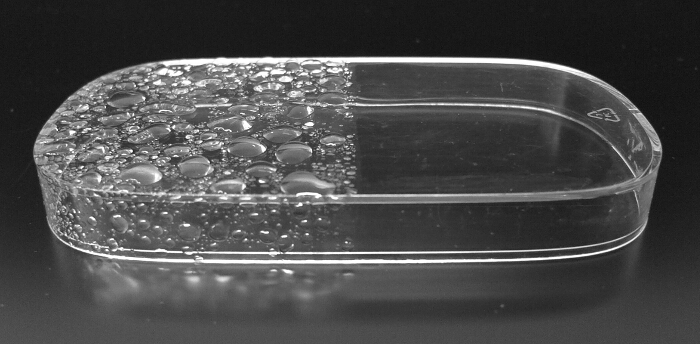
Figure 3. Demonstration of Hydrophilic Coating (1). Right half of the surface of polycarbonate plastic was coated with nanoparticles, while left half was uncoated. When the whole surface was sprinkled with a water sprayer, coated surface did not form water droplets. Please click here to view a larger version of this figure.
The coating effect can readily be visualized by comparing the wetting effect before and after the coating. Figure 3 demonstrates the hydrophilic coating effect on the surface of a plastic, polycarbonate. While the right half of the top surface was coated, the left half was intact. Sprinkling on the surface of these surfaces clearly demonstrates the coating effect. The right half forms a very thin film of water, while the left half forms water droplets showing that water does not wet the surface.

Figure 4. Demonstration of Hydrophilic Coating (2). Driver's seat window of an automobile was coated with nanoparticles while rear passenger window was uncoated. When both windows were sprinkled with a water sprayer, coated surface did not form water droplets. Please click here to view a larger version of this figure.
For additional demonstration, the produced nanoparticle was sprayed on the surface of auto glass (Figure 4). The driver's seat window was coated with nanoparticles, while the rear passenger window was not coated. When water was sprayed on both of the glass windows, the sprayed water formed a thin water layer on the coated glass while water droplets are formed on the uncoated glass. This photo clearly demonstrates that the coated glass offers much better visibility than uncoated one.
Discussion
There are several critical steps in the production of the nanoparticle suspension. If the purified gliadin contains impurities, the reaction with ECA will produce side products. Although these unwanted products can be removed from the reaction medium during the centrifugation stage, it lowers the yield of the major product. If the gliadin solution prepared during experimental step 2.3) does not show clear separation between supernatant and precipitate after two days, the solution needs to stand for longer time. Using fresh ECA is of utmost important because the shelf life of ECA is limited.
The partially polymerized ECA in old ECA produces larger size nanoparticles that are not stable in the suspension medium. The concentration of the each reactant can be modified. However, the size and stability of the final product will be affected by the modification. Therefore it is necessary to see if the produced nanoparticles form aggregates or not. An optical microscope can be used for this purpose as the size of aggregates is in the range of a few micrometers. The produced nanoparticles begin to decompose at higher than 120 °C. If the temperature of the coated surface has been raised to higher than 120 °C, it needs to be coated again. The coated surface is stable under normal conditions. Unless the coated material is exposed to harsh conditions such as heating to higher than 120 °C or immersing in alkaline water, the coating did not show any sign of degradation for several months.
The advantage of the coating process presented here is that the coating takes place very quickly because the coating process involves the fast adsorption of nanoparticles onto the glass (or other materials). The reason for adopting protein as a part of nanoparticle is that protein molecules possess both positive and negative charges in its backbone. Therefore, the hydrophilicity is not affected by the change in the pH of contacting water droplets. Coating via chemical reaction with glass is a slow process and requires toxic chemicals11 which could be a limitation of this technique. The presented coating can be applied in many different areas, such as: on glass (or Plexiglas) windows (automobile, building) for better view on rainy days; on metals for pre-treatment for chrome coating; on laparoscopes to prevent lens-fogging during laparoscopic surgery; on solar cells to prevent watermarks left after rain; and for post-treatment of janitorial work for fast drying and preventing water stains.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
Thanks to Mr. Jason Adkins for expert technical assistance.
Materials
| Ethyl cyanoacrylate (ECA) monomer | K&R International (Laguna Niguel, CA) | I-1605 | Any pure ECA can be used. |
| Gliadin | MGP Ingredients, Inc (Atchison, KS) | Gift from the company | Gliadin can be purchased from Sigma-Aldrich (cat #: G3375-25G). Instead of gliadin, any commercial gluten can be used. |
| HCl | Any | Any reagent grade chemical can be used. | |
| Acetone | Any | Any reagent grade chemical can be used. | |
| Methanol | Any | Any reagent grade chemical can be used. | |
| Ethanol (100%) | Any | Any reagent grade chemical can be used. | |
| Filter paper | Any | Any grade filter paper larger than 10 cm can be used. | |
| Cell culture square dish | Any | Any dish larger than 20 cm x 20 cm can be used. | |
| Coffee grinder | Any | Any coffee grinder can be used. | |
| Rotary evaporator | Any | Any rotary evaporator can be used. | |
| Freeze Dryer | Any | Any freeze dryer that can reach – 70°C can be used. | |
| Centrifuge | Any | Any centrifuge that can apply 1000 x g can be used. | |
| Magnetic stirrer | Any | Any magnetic stirrer that can turn spin bar to 1000 RPM can be used. | |
| Dynamic Light Scattering (DLS) | Brookhaven Instruments Corporation | NanoBrook Omni Zeta Potential Analyzer | DLS from any company can be used. |
| Scanning Electron Microscope (SEM) | Carl Zeiss Inc. | Any SEM can be used. | |
| Dynamic Contact Angle (DCA) | Thermo Cahn Instruments | Any DCA can be used. |
Referanslar
- Vauthier, C., Dubernet, C., Fattal, E., Pinto-Alphandary, H., Couvreur, P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv. Drug Deliver. Rev. 55, 519-548 (2003).
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 24, 115-119 (2007).
- Bietz, J. A., Wall, J. S. Identity of high molecular weight gliadin and ethanol soluble glutenin subunits of wheat: Relation to gluten structure. Cereal Chem. 57, 415-421 (1980).
- Kim, S. Production of composites by using gliadin as a bonding material. J. Cereal Sci. 54, 168-172 (2011).
- Kim, S., Kim, Y. Production of gliadin-poly(ethyl cyanoacrylate) nanoparticles for hydrophilic coating. J. Nanopart. Res. 16, 1-10 (2014).
- Behan, N., Birkinshaw, C., Clarke, N. Poly n-butyl cyanoacrylate nanoparticles: a mechanistic study of polymerisation and particle formation. Biomaterials. 22, 1335-1344 (2001).
- Nicolas, J., Couvreur, P. Synthesis of poly(alkyl cyanoacrylate)-based colloidal nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 1, 111-127 (2009).
- Kim, S., Evans, K., Biswas, A. Production of BSA-poly(ethyl cyanoacrylate) nanoparticles as a coating material that improves wetting property. Colloid Surface. B. 107, 68-75 (2013).
- Lander, L. M., Siewierski, L. M., Brittain, W. J., Vogler, E. A. A systematic comparison of contact angle methods. Langmuir. 9, 2237-2239 (1993).
- Davies, J., Nunnerley, C. S., Brisley, A. C., Edwards, J. C., Finlayson, S. D. Use of Dynamic Contact Angle Profile Analysis in Studying the Kinetics of Protein Removal from Steel Glass, Polytetrafluoroethylene, Polypropylene, Ethylenepropylene Rubber, and Silicone Surfaces. J. Colloid Interf. Sci. 182, 437-443 (1996).
- Giolando, D. M. Nano-crystals of titanium dioxide in aluminum oxide: A transparent self-cleaning coating applicable to solar energy. Sol. Energy. 97, 195-199 (2013).

