Immunofluorescence to Monitor the Cellular Uptake of Human Lactoferrin and its Associated Antiviral Activity Against the Hepatitis C Virus
Özet
The human lactoferrin (hLF) is a component of the immune system. In this study, immunofluorescence assays are used to demonstrate both the hepatocellular uptake of hLF and a qualitative reduction in the hepatitis C virus replication upon treatment with hLF.
Abstract
Immunofluorescence is a laboratory technique commonly used to study many aspects of biology. It is typically used to visualize the distribution and/or localization of a target molecule in cells and tissues. Immunofluorescence relies on the specificity of fluorescent-labelled antibodies against their corresponding antigens within a cell. Both direct and indirect immunofluorescence approaches can be used which rely on the use of antibodies linked with a fluorochrome. Direct immunofluorescence is less frequently used because it provides lower signal, involves higher cost and less flexibility. In contrast, indirect immunofluorescence is more commonly used because of its high sensitivity and provides an amplified signal since more than one secondary antibody can attach to each primary antibody. In this manuscript, both epifluorescence microscopy and confocal microscopy were used to monitor the internalization of human lactoferrin, an important component of the immune system, into hepatic cells. Moreover, we monitored the inhibitory potential of hLF on the intracellular replication of the Hepatitis C virus using immunofluorescence. Both the advantages and disadvantages associated with these approaches are discussed.
Introduction
Immunofluorescence is a technique that uses a fluorescence microscope to visualize the distribution and/or localization of a target molecule in a biological sample. Immunofluorescence relies on the specificity of fluorescent-labelled antibodies against their corresponding antigens within a cell1. It is typically used on tissue sections and cultured cell lines in order to analyze the distribution/localization of various biological molecules such as proteins, nucleic acids and glycans. It should be noted that immunofluorescence is often used in combination with other non-antibody methods of fluorescent staining such as the 4′,6-diamidino-2-phenylindole (DAPI) stains which are typically used to label DNA2. Moreover, this technique involves fixation of the cells which allows the analysis of cells at a specific time.
Different types of microscopes can be used to analyze immunofluorescence samples. The simplest is the epifluorescence microscope (Figure 1) for which excitation of the fluorochrome and detection of the fluorescence are done through the same light path3. Because most of the excitation light is transmitted through the sample, only reflected excitatory light can reach the objective together with the emitted light. This approach unfortunately leadsto a frequent high signal to noise ratio.In contrast, confocal microscopy (Figure 2) offers a distinct advantage for increasing optical resolution and contrast by means of adding a spatial pinhole placed at the confocal plane of the lens to eliminate out-of-focus light4. This approach allows the reconstruction of three-dimensional structures from the obtained images. However, since an important fraction of the light from the sample is blocked at the pinhole, long exposures are often required.
There are two classes of immunofluorescence techniques, primary (or direct) and secondary (or indirect). Direct immunofluorescence involves a primary antibody linked with a fluorochrome (Figure 3). This method is less frequently used because it provides lower signal, involves higher cost and less flexibility1. Moreover, such antibodies are generally harder to find commercially. On the other hand, the direct attachment of the fluorochrome to the antibody significantly reduces the number of steps in the procedure, saving time and frequently reducing non-specific background signal. This also limits the possibility of antibody cross-reactivty.
Indirect immunofluorescence involves a primary unlabelled antibody which is specific for the epitope of interest1. A secondary antibody which carries the fluorochrome then recognizes the primary antibody and binds to it (Figure 3). Although indirect immunofluorescence is more complex and time consuming than direct immunofluorescence, it is frequently used because of its high sensitivity and it also provides an amplified signal since more than one secondary antibody can attach to each primary antibody. In addition, a vast array of commercial secondary antibodies is available at affordable prices.
Hepatitis C virus (HCV) is a major public-health problem with 130-170 million individuals chronically infected worldwide. In order to halt the epidemic, therapy against HCV will need to be both effective and widely available. Studies focusing on safe and affordable natural product active against HCV have revealed the antiviral activity of the human Lactoferrin (hLF) protein which binds and neutralizes the circulating virion5. In the current study, investigation of hLF activity on the HCV subgenomic replicon system, which is independent from viral entry and shedding, revealed a distinct antireplicative activity of hLF against HCV. This manuscript presents a study in which immunofluorescence assays were performed to monitor the internalization of hLF, an important component of the immune system6, into hepatic cells. Moreover, we monitored the inhibitory potential of hLF on the intracellular replication of the Hepatitis C virus (HCV).
Protocol
1. Cells Preparation and Treatment
- In a 24-well plate, put a glass cover at the bottom of the wells. Wash each well with Phosphate Buffered Saline (PBS).
- Seed Huh-7 cells supporting HCV subgenomic replicon in each well to a density of 5 × 104 cells/well. If there is no treatment to do, the cells can be seeded at a higher density. The culture medium is Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Foetal Bovine Serum (FBS), 2mM L-glutamine, 1 mM sodium pyruvate and 250 mg/ml G-418 (to maintain the replicon).
- Incubate at 37 °C and 5 % CO2. The next day, treat the cells with 3 µM of hLF purified from human milk.
2. Paraformaldehyde/Sucrose Preparation (12%PAF and 12% sucrose)
- Put 500 ml of PBS in a beaker and heat it between 20 °C and 30 °C. Dissolve 60 g of sucrose in the PBS. Dissolve 60 g of paraformaldehyde (PAF) in the PBS/sucrose solution. Slowly add NaOH 2N (3 to 7 ml) until a clear solution is obtained.
- Adjust pH to 7.4 with HCl. Complete volume to 500 ml with PBS. Filter by gravity on Whatman filter. Before use, dilute the solution with PBS to a final concentration of 4% PAF and 4% sucrose (Storage 4 °C for 1 week maximum).
3. Immunofluorescence
- At the desired time (0 hr, 2 hr or 24 hr of treatment), wash cells twice (1 min) with PBS and fix with PBS/4% PAF/4% sucrose for 20 min at RT. The cells are typically at 30-40% confluence and 1.5X105 cells are used.
- Permeabilize the cells with 0.15% triton X-100 in PBS for 5 min at RT and block in 10% normal goat serum (NGS) in PBS for 20 min.
- Dilute the primary antibody of the protein of interest in 10% NGS (example: primary mouse anti-hLF antibody diluted 1:1,000). Apply the primary antibody to the cells.
- After 4 hr at RT or O/N at 4 °C, wash the cells three times for 5 min with 10% NGS. Stain the cells with secondary antibody labelled with fluorochrome diluted in 10% NGS (example: Fluorochrome with an excitation wavelength of 488 nm linked to an antibody anti-mouse 1: 1,000) for 1 hr at RT in the dark.
- Wash the cells once for 5 min with PBS and stain the cells with 4',6-diamidino-2-phenylindole (DAPI) (1 µg/ml) for 15 min at RT in the dark.
- Wash the cells twice for 5 min with PBS. Mount cover glasses on SlowFade mounting medium before visualization through microscopy. The cells are now ready for analysis by epifluorescence or confocal microscopy.
- Use different controls to ensure the specificity of the detection. For instance, all antibodies can be omitted in order to detect autofluorescence. The primary antibody can be omitted to determine the non-specific staining by the secondary antibody. Finally, if possible, control cells or tissues that do not express the molecule of interest can also be used.
- Visualize the samples using appropriate fluorescence microscope (epifluorescence or confocal) and filter sets appropriate for the fluorescent label used. Slides can also be stored in a slide box at < -20 °C for later examination.
4. Fluorescence Microscopy
- Keep the samples in the dark to prevent photobleaching. Turn on the light source of the chosen microscope (epifluorescence or confocal).
- Turn on the microscope. Turn on the camera for the microscope. Put the slide on the microscope for visualization. Add immersion oil on the slide to increase the numerical aperture of the objective lens.
- Select the appropriate filters. Adjust focus and appropriate objective. Adjust the shutters in order to detect the appropriate fluorochrome but prevent photobleaching. Keep overhead lights off to minimize background light when looking at sample.
- Change the filters to visualize various fluorochromes (for instance DAPI and for an excitation wavelength of 488 nm). Take pictures with the camera.
Representative Results
The ability of the hepatic Huh-7 cell line to internalize exogenously administrated hLF was monitored using immunofluorescence on a confocal miscroscope. hLF was added to the culture media and the internalization was allowed for 24 hr, after which, extracellular hLF was washed with PBS and residual membrane bound hLF was degraded with a 5 min trypsin treatment (1 ml). Cells were reseeded and allowed to re-adhere for 18 hr before immunofluorescence staining. In order to outline the cytoplasmic limits, the cells membranes were stained with wheat germ agglutinin (WGA) fluorochrome of an excitation wavelength of 488 nm conjugate. The hLF staining was achieved using an anti-hLF primary antibody and a fluorochrome with a wavelength of 633 nm linked to the secondary antibody. In order to determine the precise localization of hLF, cells were imaged following sequential 0.5 µm horizontal cross-section by confocal microscopy. Figure 4 presents an optical cross-section corresponding to mid-cell thickness. Following the addition of exogenous hLF, a significant amount of hLF was detected inside the cytoplasm of hepatocytes. No hLF was observed inside the untreated cells when using identical instrumental settings. Together, these data confirm that hepatocytes have the capacity to acquire hLF from their extracellular environment.
The ability of hLF to inhibit the intracellular replication of HCV was next monitored using epifluorescence microscopy. Huh-7 cells supporting HCV subgenomic replicon (expressing HCV NS3, NS4A, NS4B, NS5A, NS5B proteins) replication were treated with 3 µM hLF for 0, 2 or 24 hr. The double-immunofluorescence staining for hLF and HCV NS5A proteins was analyzed by epifluorescence microscopy. These results indicate that HCV subgenomic replicon staining (as measured by immunofluorescence of the HCV non-structural 5A protein) was reduced in cells treated with hLF. Figure 5 reveals a qualitative reduction of approximately 50% in HCV staining intensity after 24 hr of hLF treatment. This is an interesting observation as this subgenomic replicon system is lacking HCV E1 and E2 envelope glycoproteins, which are the commonly accepted pharmacological targets of hLF. Concomitant with the fading of HCV staining, hLF accumulation began to be detected after 2 hr, and strong hLF accumulation was observed after 24 hr.
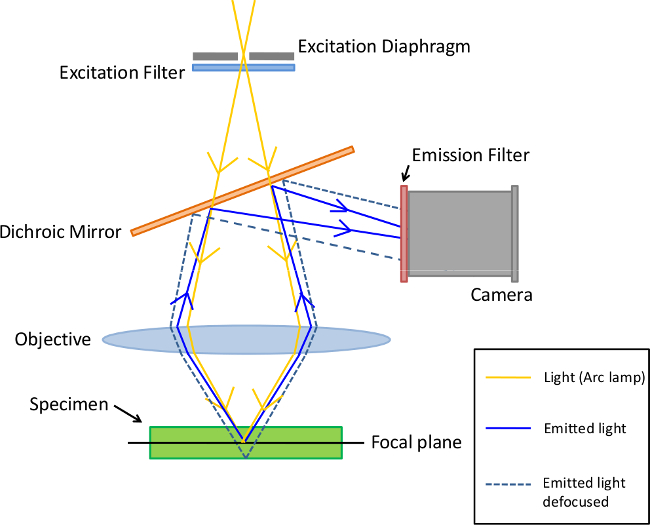
Figure 1. Principle of Epifluorescence microscopy. In this type of microscopy, excitation filters select the specific wavelength which excites the specimen (tissus or cells harboring fluorescent-labelled antibodies). Moreover, emission filters are chosen to match the spectral emission characteristics of the fluorophore used to label the specimen. Please click here to view a larger version of this figure.
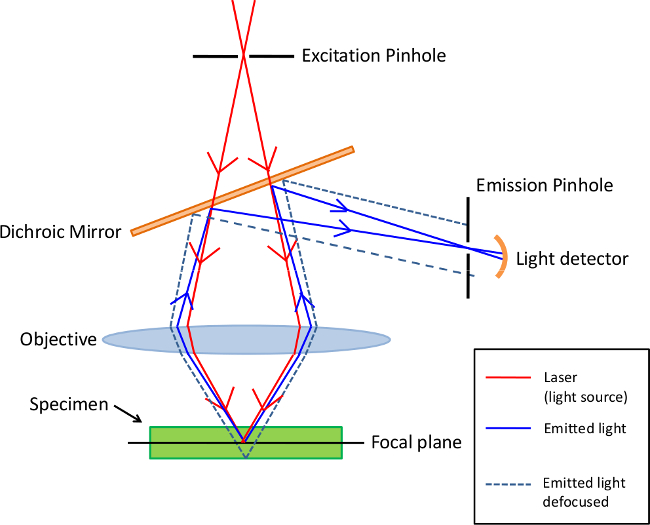
Figure 2. Principle of Confocal microscopy. This type of microscopy uses an emission pinhole to eliminate out-of-focus light signal. Since only light produced by fluorescence very close to the focal plane can be detected, the optical resolution of the image is much better than that of epifluorescence microscopes. Please click here to view a larger version of this figure.
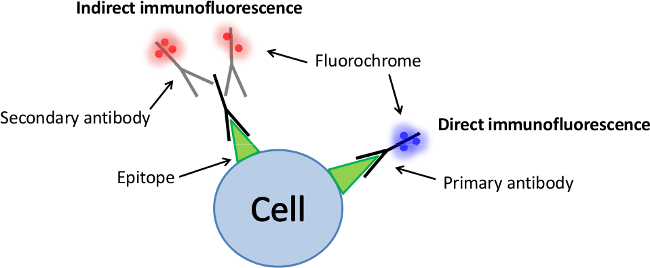
Figure 3. Schematic of the indirect and direct immunofluorescence. Direct immunofluorescence involves only one type of antibody labelled with a fluorochrome which is specific to the molecule of interest (primary antibody). In contrast, indirect immunofluorescence involves secondary antibodies labelled with fluorochromes which are specific to the species of the unlabelled primary antibody. More than one secondary antibody can attach to each primary antibody which increases the fluorescence signal. Please click here to view a larger version of this figure.
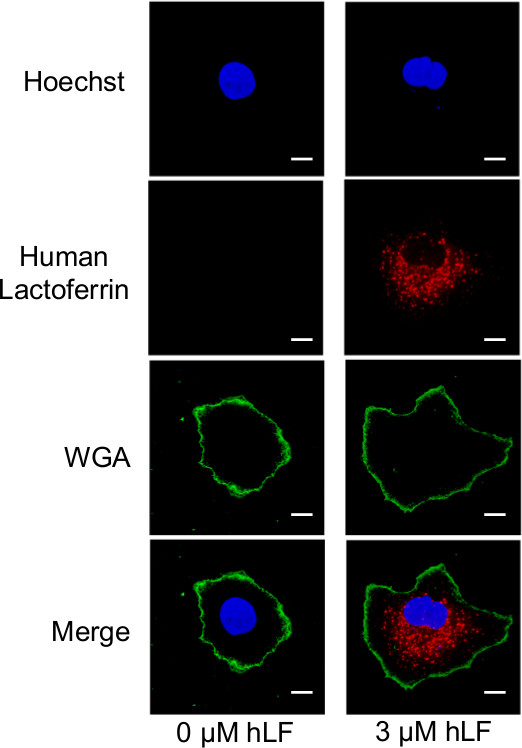
Figure 4. Hepatocellular uptake of exogenous hLF. Huh-7 cells were treated with 0 µM or 3 µM hLF for 24 hr, washed twice, submitted to a 5 min trypsin treatment, allowed to re-adhere for 18 hr and subjected to immunofluorescence. Nuclei were stained with DAPI (blue channel), plasma membranes were labeled with WGA (green channel) and hLF was stained with an anti-hLF antibody (red channel). Images were acquired by confocal microscopy at an original magnification of 60x. The horizontal optical cross-sections represent the mid-thickness of the cells. Scale bar, 10 µm. Modified from (11). Please click here to view a larger version of this figure.
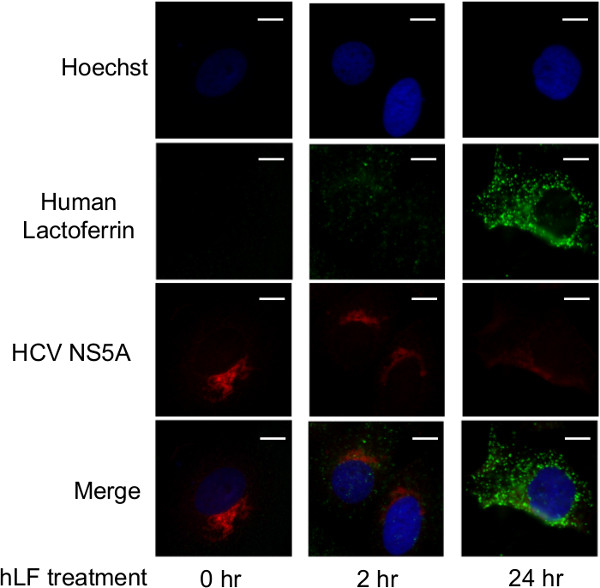
Figure 5. hLF treatment reduces HCV staining. Huh-7 cells supporting HCV subgenomic replicon replication were treated with 3 µM hLF for 0, 2, or 24 hr and subjected to immunofluorescence. Nuclei were stained with DAPI (blue channel), HCV was revealed by an anti-NS5A antibody (red channel) and hLF was stained with an anti-hLF antibody (green channel). Images were acquired by epifluorescence microscopy at an original magnification of 60x. Scale bar, 10 µm. Modified from (11). Please click here to view a larger version of this figure.
Discussion
The HCV epidemic remains a global threat, with 80% of newly infected patients developing a chronic infection, putting them at risk of cirrhosis, liver failure or hepatocellular carcinoma. Direct-acting antiviral agents targeting HCV replication and maturation represent prime anti-HCV agents, as recently demonstrated by the regulatory approval of two NS3 N-terminus protease inhibitors (Boceprevir and Telaprevir). The hLF anti-HCV activity is currently believed to behave as a viral entry inhibitor, binding circulating virions and preventing them from entering into their hepatocellular target. Our research on hLF presents a novel intracellular anti-HCV mechanism of action (distinct from extracellular virion neutralization)7.
Immunofluorescence can be efficiently used to visualize the distribution and/or localization of a target molecule in a biological sample. As found in many other fluorescence techniques, one of the main problems associated with immunofluorescence is photobleaching (photochemical destruction of the fluorochrome)8. This loss of activity caused by photobleaching can be circumvented either by employing more robust fluorochromes that are less prone to photobleaching or by reducing the intensity or time-span of light exposure. Moreover, since antibodies cannot cross the cell membrane, immunofluorescence is generally only limited to fixed cells. Alternative approaches to monitor the distribution/localization of proteins in live cells therefore generally rely on the expression of proteins containing fluorescent protein domains such as the green fluorescent protein (GFP)9. However, the addition of such GFP epitopes is sometimes associated with a modification in the localization and activity of proteins. Immunofluorescence can also be used on a number of different samples including tissue sections, cultured cell lines, or individual cells it is also frequently used to monitor the localization/distribution of proteins, glycans, and small molecules. The main advantages of this technique reside in its simplicity and the fact that it can be used in combination with other, non-antibody methods of fluorescent staining (e.g., DNA staining). Moreover, commercially produced secondary antibodies are relatively inexpensive and available in an vast array of colors.
In the current study, immunofluorescence of hepatic cells supporting an HCV subgenomic replicon revealed a qualitative reduction in the HCV staining intensity upon treatment with hLF. This is an interesting observation as this subgenomic replicon system is lacking HCV E1 and E2 envelope glycoproteins, which are the commonly accepted pharmacological targets of hLF. Unlike the extracellular virion neutralization activity, hLF would need to be internalized by HCV-infected hepatocytes in order to impair viral replication. The use of fuorescence microscopy allowed us to evaluate this possibility. Upon pre-incubation on HCV-infected hepatocytes, exogenous hLF was successfully internalized, and shown to impair intracellular viral replication. The understanding of this novel hLF antiviral activity constitutes a step forward in the comprehension of the global (and likely pleiotropic) mechanism of inhibition of this innate immunity protein.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This work was funded by both the Canadian Institutes of Health Research and Natural Sciences and the Engineering Research Council of Canada. M. Bisaillon is a Chercheur Boursier Senior from the Fonds de Recherche en Santé du Québec and also a member of the Centre de Recherche Clinique du Centre Hospitalier Universitaire de Sherbrooke. We thank Dr. Ralf Bartenschlager for the generous gift of the HCV replicon system. We also thank Dr. Charles Rice and Dr. Daniel Lamarre for kindly providing the hepatic cell line. We also want to thank Guillaume Tremblay for technical assistance.
Materials
| DMEM | Wisent | 319-005-CL | |
| PAF | BioShop | PAR070.1 | Flammable solid, skin irritant, lungs and eyes. |
| PBS | Wisent | 311-425-CL | Without Ca2+ & Mg2+ |
| NGS | Wisent | 053-150 | |
| AlexaFluor 488-labeled anti-mouse | Invitrogen | A11017 | |
| AlexaFluor 568-labeled anti-rabbit | Invitrogeb | A21069 | |
| Wheat germ agglutinin Alexa Fluor 488 conjugate (WGA) | Invitrogen | W11261 | Potentially mutagenic |
| Anti-NS5A rabbit | Abcam | ab2594 | |
| Anti-hLF mouse | Abcam | ab10110 | |
| SlowFade | Invitrogen | S36937 | |
| Hoechst stain | Life Techn. | H1399 | Potentially mutagenic and carcinogenic |
| hLF | Sigma | L0520 | |
| Nikon Eclipse visible/epifluorescence Microscope | Nikon | TE2000-E | |
| epifluorescence/confocal microscope | Olympus | FV1000 |
Referanslar
- Odell, I. D., Cook, D. Immunofluorescence techniques. J Invest Derm. 133 (1), e4 (2013).
- Portugal, J., Waring, M. J. Assignment of DNA binding sites for 4’,6-diamidine-2-phenylindole and bisbenzimide (Hoechst 33258). A comparative footprinting study. Biochim Biophys Acta. 949 (2), 158-168 (1988).
- Lichtman, J. W., Conchello, J. -. A. A. Fluorescence microscopy. Nat Methods. 2 (12), 910-919 (2005).
- St Croix, C. M., Shand, S. H., Watkins, S. C. Confocal microscopy: comparisons, applications, and problems. BioTechniques. 39, S2-S5 (2005).
- Yi, M., Kaneko, S., Yu, D. Y., Murakami, S. Hepatitis C virus envelope proteins bind lactoferrin. Journal of virology. 71, 5997-6002 (1997).
- Wakabayashi, H., Oda, H., Yamauchi, K., Abe, F. Lactoferrin for prevention of common viral infections. J Inf Chem. 20 (11), 666-671 (2014).
- Picard-Jean, F., Bouchard, S., Larivée, G., Bisaillon, M. The intracellular inhibition of HCV replication represents a novel mechanism of action by the innate immune Lactoferrin protein. Antiviral research. 111, 13-22 (2014).
- Johnson, G. D., Davidson, R. S., McNamee, K. C., Russell, G., Goodwin, D., Holborow, E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 55 (2), 231-242 (1982).
- Green Remington, S. J. fluorescent protein: a perspective. Protein Sci. 20 (9), 1509-1519 (2011).

