A Method to Study the Impact of Chemically-induced Ovarian Failure on Exercise Capacity and Cardiac Adaptation in Mice
Özet
Two exercise paradigms were tested on a newly developed chemically induced menopausal mouse model to examine the impact of menopause on exercise capacity and cardiac adaption to exercise.
Abstract
The risk of cardiovascular disease (CVD) increases in post-menopausal women, yet, the role of exercise, as a preventative measure for CVD risk in post-menopausal women has not been adequately studied. Accordingly, we investigated the impact of voluntary cage-wheel exercise and forced treadmill exercise on cardiac adaptation in menopausal mice. The most commonly used inducible model for mimicking menopause in women is the ovariectomized (OVX) rodent. However, the OVX model has a few dissimilarities from menopause in humans. In this study, we administered 4-vinylcyclohexene diepoxide (VCD) to female mice, which accelerates ovarian failure as an alternative menopause model to study the impact of exercise in menopausal mice. VCD selectively accelerates the loss of primary and primordial follicles resulting in an endocrine state that closely mimics the natural progression from pre- to peri- to post-menopause in humans. To determine the impact of exercise on exercise capacity and cardiac adaptation in VCD-treated female mice, two methods were used. First, we exposed a group of VCD-treated and untreated mice to a voluntary cage wheel. Second, we used forced treadmill exercise to determine exercise capacity in a separate group VCD-treated and untreated mice measured as a tolerance to exercise intensity and endurance.
Introduction
The natural onset of menopause is characterized by the depletion of ovarian primordial follicles through atresia, eventually resulting in ovarian senescence. In the United States, over 30% of a women's lifetime will be spent post-menopausal. In response to a decrease in ovarian function, several physiological and psychological consequences may occur, including increased adiposity, vascular instability, and mood or sleep disturbances. Because of increased obesity/adiposity compared with premenopausal counterparts1,2, postmenopausal women are particularly more susceptible to metabolic syndrome and associated comorbidities, including cardiovascular disease (CVD)3. Moreover, it is becoming increasingly evident that successful cardiac rehabilitation for patients with CVD includes regular aerobic exercise, and that exercise reduces cardiovascular morbidity and mortality in these subjects4,5. Yet, how exercise capacity changes during the pre- to post-menopausal transition has not been well studied. More importantly, the role of exercise as a preventative measure for CVD risk in post-menopausal women remains remarkably understudied.
The most commonly used inducible model for mimicking menopause in women is the ovariectomized (OVX) rodent. Recently, the occupational chemical 4-vinylcyclohexene diepoxide (VCD) has been shown to specifically target small primary and primordial ovarian follicles by accelerating the natural process of atresia, ultimately resulting in ovarian failure with no obvious toxicity found in other tissues6. The estrous cycle of VCD-treated mice, which is analogous to a human menstrual cycle, ceases within 2-3 months after the completion of VCD injections, which in turn results in a gradual withdrawal of estrogen, mimicking the peri- to post-menopausal transition. Thus, a follicle-deplete, ovary-intact animal closely approximates the natural human progression through the pre- to peri- to post-menopausal transition7-9. Moreover, it provides a nonsurgical alternative to the OVX model reducing complications from surgery, such as infection. In this study, we employed VCD-induced menopausal mice to study the impact of exercise on the cardiac adaption in menopausal mice.
There are several instances that suggest the association between estrogen and cage wheel exercise in rodents of both sexes10-13. Estrogen depletion by surgical OVX decreases voluntary exercise activity in mice and rats14,15. Two methods of exercise were used in this study to test the exercise capacity of VCD-induced menopausal mice. Cage wheel running is generally regarded as a type of voluntary exercise in animal models and performed, presumably, under less stressful conditions. However, cage wheel running is not indicative of the relative exercise capacity of animals, which requires animals to exercise at much higher levels. Forced treadmill exercise was used to determine exercise capacity, measured as a tolerance to exercise intensity and endurance. In addition, we have previously shown that cage wheel exercise provides a stimulus for cardiac hypertrophy and this hypertrophic growth is sex-dependent16. Therefore, we also measured cardiac adaption to cage wheel exercise in VCD-induced menopausal mice.
Protocol
All experiments, housing, and caring for animals was performed using protocols that adhered to guidelines and approved by the Institutional Animal Care and Use Committee at the University of Arizona, and to 2011 NIH guidelines for care and use of laboratory animals.
1. Treatment with 4-Vinylcyclohexene Diepoxide
- VCD dosing protocol: In a chemical fume hood, add 0.587 ml of VCD to a clean flask, bring the final volume to 10 ml with sesame oil, and then mix by inverting gently, cover and seal with Parafilm. Transfer the solution to a dosing vial, seal it with Parafilm, and store at 4 °C for up to 7 days. Be sure all reagents are sterile.
- Use sesame oil as the vehicle control as VCD is dissolved in sesame oil. Store VCD (MW=140.2 g/mol, density=1.09 g/ml) at -20 °C.
- VCD injection protocol: At 2 months of age, weigh and administer daily intraperitoneal injections of room temperature VCD at a dose of 160 mg/kg for 20 consecutive days. Inject control mice in a similar manner with the vehicle solution, sesame oil, at a volume of 2.5 ml/kg.
Note: During the injection period, try to alternate the sites of injection to reduce the chances of local infection. Use the following equation to calculate the volume needed for injection: Body weight (g) x kg/1,000 g x 2.5 ml/kg = volume (ml) of solution to inject resulting in approximately 50 ml/injection using a 27 G needle. Due to the size of the needle local anesthetics are not needed. - Estrous cycle monitoring: After the injection period is complete, monitor the estrous cycles daily by obtaining vaginal smears. Restrain mice using the dorsum scruff method. Insert an eyedropper with 0.2 ml of sterile saline into the vaginal orifice to a depth of 0.5 cm. Triturate saline (3x) and place 1 drop onto a blank slide. Immediately evaluate samples microscopically using a standard light microscope at 100X magnitude.
Note: Vaginal cytology is determined to ensure loss of cycling and therefore validate ovarian failure. Figure 2A illustrates typical vaginal cytology across different phases of the estrous cycle in mice, namely proestrus, estrus, metestrus, diestrus17-19. During proestrus, cells include nucleated epithelial cells (Figure 2A, arrows indicate nucleated cells); during estrus, cells are predominantly cornified squamous epithelial cells; Metestrus is characterized by a mix of cell types, with a predominance of leukocytes; Diestrus is dominated by leukocytes. Adjustment of the focal plane may be required to visualize cells and nuclei throughout the sample. As illustrated in Figure 2B, a typical murine estrus cycle is 4-5 days. Mice are considered acyclic after 15 consecutive days in persistent diestrus. Continuously monitor estrous cycles throughout the duration of the study period to confirm lack of estrous cycles in VCD treated mice.
2. Voluntary Cage-wheel Exercise
Inbred C57BL/6 and B6C3F1 female mice, aged 7 months, are exposed to voluntary cage wheel running. House animals individually in a cage (47 cm x 26 cm x 14.5 cm) with unencumbered access to a cage wheel for 28 days.
- Equipment setup: The cage wheel apparatus consists of an 11.5 cm diameter wheel with a 5.0 cm wide running surface equipped with a digital magnetic counter that is activated by wheel rotation. Before each wheel running test, calibrate the cage wheel according to the manufacturer's guidelines. Measure the inner diameter of the running wheel, calculate the circumference and enter this value into the digital counter as wheel size.
- Attach the computer portion of the digital magnetic counter to the wheel stand using tape.
- Attach the computer portion of the digital magnetic counter to the wheel stand using tape.
- Attach the free magnet of the digital magnetic counter to the central bar on the same side as the magnet cabled to the computer, again taping sufficiently to protect the magnet from chewing damage. The magnets should be positioned so that each rotation of the wheel is recorded by the computer without any interference or resistance to complete wheel rotation.
Note: Duct tape has been used successfully to attach the components of the digital magnetic counter to the running wheel. Taping should be sufficient to completely cover the magnet and prevent damage from chewing by the animal. The magnet should be positioned as close as possible to the wheel without hindering its movement. However, the setup should be monitored daily for chewing damage and repaired as necessary. - Mount the wheel to the cage by sliding the wheel stand through the top wiring of the animal cage. Secure the wheel stand to the cage wiring with binder clips.
Note: The clip should be on the top of the wiring, away from the animal. Feed the computer portion and any extra cable through the wiring of the cage. Place the computer and excess cable out of reach of the animal. This may be accomplished by placing a medium-sized plastic weigh dish on top of the wiring of the cage to hold the computer and excess cable.
- Exercise: For a given litter, randomly assign mice to either the sedentary control or exercise regimen. Give all animals water and standard hard rodent chow ad libitum.
- Manually record daily values for the distance ran (km/day) and total time spent running (hr/day), given by the magnetic counter. Record distance and time values within the same 1 hr time frame every day for accurate and consistent values throughout the 28 day duration of the exercise period. Clear (zero) the magnetic counter after each day's recording to ensure accurate readings the following day.
- Calculate the average values of distance and speed for each group. Calculate speed based on the given values of distance and time each day for each mouse. Data can be displayed as daily or weekly averages for each measured parameter.
- Tissue harvest: At the end of the specific exercise period, euthanize exercised and sedentary control animals within 30 min of removal from cages by cervical dislocation under inhaled anesthesia. Weigh body mass and rapidly excise hearts, skeletal muscle, and ovaries.
- At the time of tissue harvesting, mount and stain the collected ovaries with hemotoxylin and eosin (H&E; Figure 2C). Under magnification, count and classify follicles as primordial, primary, secondary, or antral for each VCD-treated and control animal20. A primordial follicle contains a small primary oocyte, a single layer of flattened or squamous granulosa cells closely opposed to the oocyte, and a basal lamina. A primary follicle is defined by the presence of one or more cuboidal granulosa cells that are arranged in a single layer surrounding the oocyte. A secondary follicle contains granulosa cells that form multiple layers around the oocyte, and a theca. An antral follicle is characterized by a cavity or “antrum” containing fluid.
- To excise the heart, make a medial incision on the ventral side and visualize the heart after opening the diaphragm. Rapidly excise the heart at the base and place in cold phosphate buffered saline to sufficiently wash-out residual ventricular blood. Trim the heart of excess tissue, weigh the whole heart and snap-freeze in liquid nitrogen.
- Wash all other excised tissues with a modified ice-cold phosphate-buffered saline solution (PBS), and snap-freeze in isopentane cooled in liquid nitrogen.
3. Forced Treadmill Exercise
- When employing a forced exercise paradigm to measure exercise capacity, use a treadmill and set up as follows:
- Place an electrical shock grid at the base of the treadmill ramp. This shock generated serves as a stimulus for exercise. The intensity and frequency of the electrical stimuli are controlled manually by the users. Grids can be enabled or disabled individually for each lane. All data collection is done manually.
- Periodically, calibrate the treadmill according to the manufacturer's guidelines.
- Ensure that the Treadmill Controller front panel toggle is set to “STOP”.
- Place speed sensor on treadmill belt lane1 and securely fasten with screw nut in position shown below.
- Using the dial on the Treadmill Controller front panel, adjust the speed to 88.7 m/min Then, set the electric stimulus frequency to 2 Hz on Treadmill Controller front panel.
- Push the “ODOMETER RESET” button to initialize calibration procedure.
Note: Sedentary or nonexercise control groups must be included in the experimental design. The sedentary group is age matched to the exercise group. We have previously shown that housing the sedentary mice individually in cages of equal size to the exercised mice did not impact the parameters of interest when compared to sedentary mice housed with siblings. Therefore, sedentary mice were kept with siblings in the same room as the exercised mice.
- Acclimation: Acclimate mice to the treadmill 3-7 days prior to the experiment daily. To do this, place mice in the treadmill with the belt unmoving and shock grids off, but with the belt motor on (noise). Leave mice undisturbed for at least 15 min.
- Warm up: Warm up steps as used in this protocol are as follows:
- Place each mouse on the treadmill. Disable the shock grid and increase belt speed to 4 m/min for 15 min.
- Enable the shock grid and maintain belt speed at 4 m/min belt speed for an additional 10 min.
- Exercise training regimens
- Following warm up, keep shock grids on and increase belt speed to 5 m/min for 15 min.
- Increase belt speed to 15 m/min for an additional 15 min.
- Following training, transfer mice back to their cages.
Note: In this study, exercise training was performed daily for 1 week. - Acute exercise regimen: An acute exercise regimen is designed to determine the short and long term effects of extreme exercise stress.
- Following warm up, increase belt speed from 5 m/min to 10, 15, 20, 25, and 30 m/min in 10 min intervals.
Note: Terminate exercise if mice are unable to continue running or show signs of exhaustion or distress. In our study, most animals were stopped at 25 m/min, and no animal passed the 30 m/min stage. - Test each mouse three times, allowing 2-3 days between each test. Calculate average values across all exercise sessions for each mouse.
- Following warm up, increase belt speed from 5 m/min to 10, 15, 20, 25, and 30 m/min in 10 min intervals.
- Exercise regimen for endurance: An endurance exercise protocol is designed to train mice for an extended exposure to the treadmill exercise stimulus. This type of protocol tests the capacity to tolerate the long-term effects of moderate exercise stress.
- Begin with the belt speed outlined during the warm up period, 4 m/min.
- Gradually increase belt speed by 1m/min until a final speed of 20 m/min is reached. Total duration of a typical study protocol is 50 min.
- After exercise regimen, transfer mice back to their cages.
Note: Running mice to exhaustion is avoided. If at any point during the experiment a mouse shows signs of exhaustion, deactivate the shock grid and allow the mouse to rest and recover.
4. Data and Statistical Analysis
- Present results as mean ± SEM and use 2-way ANOVA (treatment group and exercise as main factors) followed by a Student-Newman Keuls post hoc test or a student's t test to compare differences between mean values.
- Determine the percent change in cardiac mass with exercise by comparing the mass of each exercised animal to the mean cardiac mass of the sedentary group. The difference in cardiac mass is then expressed as a percent change from sedentary animals for each respective animal. p values of <0.05 are considered statistically significant.
Representative Results
A typical experimental protocol, such as that used in this study, is illustrated in Figure 1. Following 20 consecutive days of VCD treatment, vaginal cytology was used to determine the presence or absence of cyclicity in the VCD-injected mice; vehicle-injected mice were also monitored. The stage in the estrus cycle was determined by the proportion of epithelial cells, cornified epithelial cells, and leukocytes in the vaginal smear (Figure 2A). The presence of only cornified epithelial cells indicated the mouse was in estrus, and therefore was still cycling. The estrous cycle in mice typically lasts 4 days, therefore VCD injected animals were considered acyclic after 15 days of persistent diestrus, in which vaginal cytology revealed a majority of leukocytes (Figure 2A). The estrous cycle of VCD-treated mice became irregular, and then ceased within 2-3 months after the start of VCD injections (Figure 2B). VCD ovarian tissue showed significant atrophy, compared to controls. At high magnification, we see a complete depletion of primary and primordial follicles (Figure 2C).
To test the effect of VCD-induced ovarian failure on exercise performance, we randomized 7 month old VCD- and vehicle-treated mice (two strains, C57BL/6 and B6C3F1) to either sedentary or cage wheel exercise groups 4-6 weeks following the cessation of cycling (Figure 1). Daily exercise values were recorded for time and distance for each exercised animal throughout the 4 week exercise period. Weekly averages of daily running values for distance, time and speed are shown in Figure 3 for C57BL/6 mice treated with vehicle or VCD. We did not see significant differences in exercise performance measured by average daily time, distance, and speed on the cage wheel between VCD- and vehicle-treated groups in either C57BL/6 or B6C3F1 mouse strain (Figure 4A). The calculated wheel-running speeds gradually increased over the 4-week running period as previously shown16, but were not significantly different between experimental groups. These studies indicate the impact of VCD-induced ovarian failure on voluntary cage wheel exercise transcends at least 2 mouse strains.
Following the duration of the voluntary cage wheel exercise protocol, body morphometrics were recorded and the hearts were rapidly excised and weighed. We have previously shown that voluntary cage wheel exercise induces an increase in cardiac mass16. Here, we also show cardiac hypertrophy in response to cage wheel exercise measured by absolute heart weight (HW) and HW normalized to tibial length (TL) (Table 1). The absolute heart mass and HW/TL ratio were significantly greater in vehicle- and VCD-treated mice when compared to sedentary counterparts. However, there were no measurable differences in cardiac hypertrophy between control and VCD-induced ovarian failure mice following voluntary cage wheel exercise.
Next, we used involuntary (forced) treadmill running to determine exercise capacity. We first subjected mice to a high-intensity protocol that increased average speed stepwise every 10 min until exhaustion. On average, the maximal speed of vehicle treated mice was 26.7± 0.95 m/min and the maximal speed of VCD treated mice was 28±1.4 m/min before exhaustion. We used lower-intensity running at 20 m/min (about 80% of maximal) to assay for endurance capacity. As shown in Figure 4B, no significant difference in high or low intensity exercise capacity was found between vehicle and VCD treated group, indicating that VCD-induced ovarian failure had no effect on mouse exercise capacity.
In conclusion, menopause induced by VCD treatment has no effect on either voluntary or forced running capacity, as well as the cardiac adaptive response to exercise.
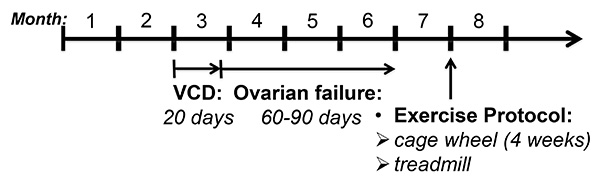
Figure 1. VCD-administration and experimental protocol. Mice were administered VCD (160 mg/kg) or sesame oil (vehicle control) starting at 2 months of age for 20 consecutive days. Following VCD or vehicle injections, estrous cycles were monitored and cyclicities were determined as described in the Methods section. Loss of ovarian function occurred within 60-90 days and was confirmed by vaginal cytology. At 7 months of age, mice were exposed to voluntary cage wheel exercise for 4 weeks or subjected to the treadmill exercise protocol.
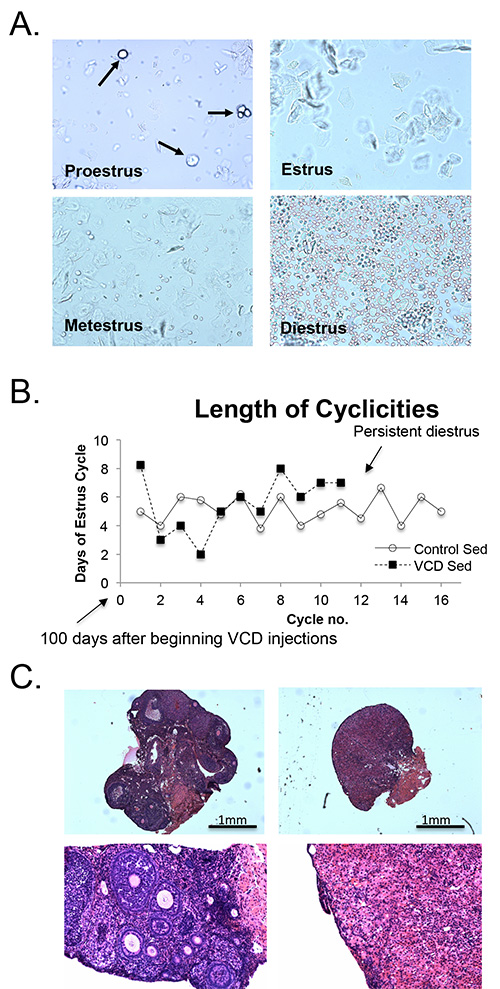
Figure 2. VCD-induced ovarian failure in mice. A: Cytology of an unstained vaginal smear from a vehicle injected mouse demonstrating the four stages of the estrous cycle: 1) proestrous, predominantly consisting of nucleated epithelial cells 2) Estrus, characterized by un-nucleated cornified cells; 3) metestrus, consisting of nucleated epithelial cells (arrows), un-nucleated cornified epithelial cells (arrows), and leukocytes; 4) diestrus, consisting primarily of leukocytes; and. B: Average length (days) of the estrous cycle of vehicle- and VCD-injected groups. One cycle is measured from the first day in estrus to the first day of the next period in estrus. Vaginal cytology began approximately 6 weeks following the onset of injections. The estrous cycles of VCD-injected animals become irregular, compared to vehicle-injected animals, and after 12 estrus cycles, cytology of all VCD injected animals no longer indicated cycling. C: H&E staining of ovarian tissue at 8 months of age. Cross-sections done at 5μm thickness for vehicle-treated (left panel) and VCD-treated (right panel) mice at 20X (top panel) and 200X (bottom panel) magnification. Ovarian tissue at 200X magnification shows a number of follicles in vehicle- compared to VCD-treated mice. Please click here to view a larger version of this figure.
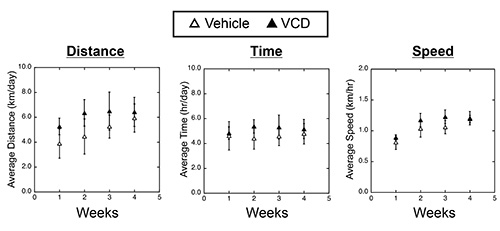
Figure 3. Voluntary cage wheel performance in vehicle- or VCD-treated C57Bl/6J female mice. Left Panel: Average running distance (km/day) for every 1 week period over the 4 week study period. Middle Panel: Average running time (hr/day) for every 1 week period over the 4 week study period. Right Panel: Average running speed (km/hr) for every 1 week period over the 4 week study period. Please click here to view a larger version of this figure.
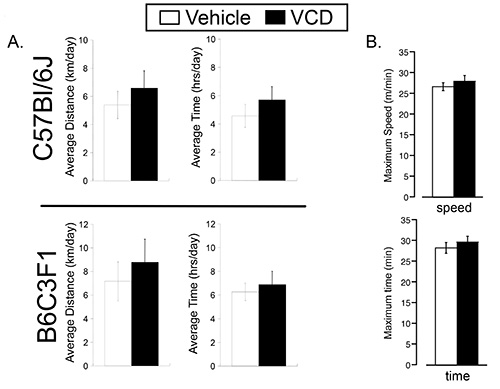
Figure 4. Voluntary cage wheel and treadmill performance in vehicle- and VCD-treated mice. A: (Top Panel) C57BL/6 average running distance (km/day) and time spent on the wheel (hrs/day) for every 24 hr over the 4 week study period. (Bottom Panel) B6C3F1 average running distance (km/day) and time spent on the wheel (hr/day) for every 24 hr over the 4 week study period. B: C57BL/6 treadmill running parameters. Maximum time (Top Panel) and maximum speed (Botoom Panel) using protocols detailed in the methods section.
Discussion
Both cage wheel exercise and treadmill running exercise regimens have been used in our previous studies16,21,22. All animals are kept in dedicated exercise rooms to minimize environmental or human disturbances. Any disturbance of the animals' biological rhythm can significantly affect animals' exercise performance. Therefore, the entire animal care, performance monitoring and recording, and exercise regimens should be done within the same daily time frame. Investigators should strive to avoid extra stress to the animals by exhibiting precautions such as limiting the duration of stress exposure and covering the cages when appropriate.
Cage wheel exercise
When preparing the cage wheel apparatus, the wheel should be easily accessible for the mouse to mount and exit. There should minimal bedding at the bottom of the cage to prevent mice from nesting around the wheel and hindering free wheel movement. The magnetic sensor was placed on the stationary portion of the wheel and the magnet secured to the free wheel, so as to count each rotation of the wheel as the mouse exercised. The recording device attached to the magnetic sensor should be placed above the wire lid of the cage, and all exposed wires need to be either taped or kept out of reach from the mice, so as to provide a barrier against chewing damage. The magnet and magnetic sensor were also taped to the appropriate structures to prevent both damage and unwanted movement. During daily data collection, all the taped wires must be closely checked to insure the wires remain intact and should be retaped as necessary. Since it may require several days for mice to acclimatize to the new environment and begin exercising regularly, data recording should be tailored to each individual animal. Although animals tend to spend many hr on the wheel, most of the running occurs at night. Therefore, some animals may reveal high performance activity even if they appear dormant during daytime. Furthermore, it is not uncommon that some animals will exhibit limited cage wheel running. Typically, cage wheel activity that amounts to an average 1 km/day or less is the result of random cage activity and not associated with any significant running stimulus. Therefore, animals whose running parameters are at 1 km/day or 1 hr/day or less should be excluded in the final data analysis nonrunners.
Depending on the specific purposes of an individual study and strains of mice involved, the length of exercise can be modified. Our previous study showed that for both mouse strains involved, the average wheel-running speeds and the duration of exercise on the cage wheel gradually increased over time and plateaued by 4 weeks.
Cage wheel running is a type of voluntary exercise in animal models, which is generally taken as being performed under less stressful conditions. Unlike treadmill exercise in which certain parameters can be preset, all running in the test cage is voluntary and easily affected by environmental or behavioral cues. Therefore, the comparisons between different experimental settings are typically avoided unless large experimental groups numbers are used. Other potential areas of research that can benefit from this type of exercise paradigm are studies that incorporate behavioral responses with physiological consequences such as cognitive neuroscience. For studies utilizing the voluntary cage wheel paradigm, the most critical steps are cage set up and monitoring of the exercise activities.
Treadmill exercise
Acclimation steps are necessary to prepare animals for the treadmill exercise protocol. Depending on the specific purpose of the individual study, the duration of the acclimation period can vary. In this study, a period of one week was chosen to allow animals to acclimate to the treadmill apparatus and motor noise in the cage, warm up periods are an important step of the exercise training regimen. Similar to human counterparts, the mice must be warmed up before getting into any intensive exercise regimens to avoid injury and potential data artifacts. In this study, we began the belt at the lowest speed possible (4 m/min) with the shock grids turned off for 15 min. All mice involved started to run on and off the belt. The shock grids were then turned on and the belt speed maintained at 4 m/min. To avoid over-stressing the mice, we set the electric shock at a mild level (1 Hz). Within a few days, all mice adapted to the treadmill belt.
The duration of running as well as the acceleration can also be tailored to each experimental design. A recommended starting acceleration rate is 1 m/min. The treadmill lanes of mice showing signs of exhaustion must be turned off immediately. Typically signs of exhaustion exhibited by mice on the treadmill are as follows:
- Continuous exposure to the shock grid for >5-10 sec,
- Loss of running capacity resulting in shock grid exposure >5x during a single exercise regimen, and
- Loss of running capacity resulting in shock grid exposure >2 sec at least 3x during a single exercise regimen.
The values listed above are to be used primarily as guidelines for a specific experiment. These parameters need to be determined for every exercise protocol as it varies from mouse strain to mouse strain, experiment to experiment, and investigator to investigator.
Unlike cage wheel exercise, treadmill studies must be repeated several times to eliminate the effects of environmental and biological factors on running performance. It is not uncommon to see fluctuations in running performance between tests even for the same animal. Since each exercise regimen exposes running mice to a stress, an appropriate resting interval between tests is necessary. In our experience, 2-3 days resting period between each test was sufficient for mice to recover.
Like human treadmill exercise, the speed for endurance exercise is best determined by the heart rate or blood oxygen saturation, a technically difficult feat with mouse studies. The most critical step of treadmill studies is determination of exhaustion, which usually varies between individual mice, and was set as 80% maximal speed. In our own experience the recording of results may significantly differ between individual researchers, since the criteria for exhaustion are mostly subjective. Thus, the consistent application of the same criteria during the recording is critical.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH grant (HL 098256), by a National and Mentored Research Science Development Award (K01 AR052840) and Independent Scientist Award (K02 HL105799) from the NIH awarded to J.P. Konhilas and the Interdisciplinary Training Grant in Cardiovascular Sciences (HL007249). Support was received from the Sarver Heart Center at the University of Arizona and from the Steven M. Gootter Foundation.
Materials
| 4-vinylcyclohexene diepoxide (VCD) | Sigma, cat# V-3630 | ||
| 11.5-cm-diameter wheel with a 5.0-cm-wide running surface | Petsmart,model 6208 | ||
| Digital magnetic counter | Sigma Sport,model BC 600 | ||
| Treadmill exercise | Columbus Instruments , model 1055SDRM |
Referanslar
- Lindheim, S. R., et al. Comparison of estimates of insulin sensitivity in pre- and postmenopausal women using the insulin tolerance test and the frequently sampled intravenous glucose tolerance test. J. Soc. Gynecol. Invest. 1, 150-154 (1994).
- Dubnov-Raz, G., Pines, A., Berry, E. M. Diet and lifestyle in managing postmenopausal obesity. Climacteric. 10 Suppl 2, 38-41 (2007).
- Grundy, S. M. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J. Am. Coll. Cardiol. 47, 1093-1100 (2006).
- Sharma, S., Firoozi, S., McKenna, W. J. Value of exercise testing in assessing clinical state and prognosis in hypertrophic cardiomyopathy. Cardiol. Rev. 9, 70-76 (2001).
- Guyatt, G. H., Devereaux, P. J. A review of heart failure treatment. Mt. Sinai. J. Med. 71, 47-54 (2004).
- Mayer, L. P., et al. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod. Toxicol. 16, 775-781 (2002).
- Mayer, L. P., Devine, P. J., Dyer, C. A., Hoyer, P. B. The follicle-deplete mouse ovary produces androgen. Biol. Reprod. 71, 130-138 (2004).
- Lohff, J. C., Christian, P. J., Marion, S. L., Hoyer, P. B. Effect of duration of dosing on onset of ovarian failure in a chemical-induced mouse model of perimenopause. Menopause. 13, 482-488 (2006).
- Wright, L. E., et al. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J. Bone Miner Res. 23, 1296-1303 (2008).
- Ogawa, S., Chan, J., Gustafsson, J. A., Korach, K. S., Pfaff, D. W. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 144, 230-239 (2003).
- Roy, E. J., Wade, G. N. Role of estrogens in androgen-induced spontaneous activity in male rats. J. Comp. Physiol. Psychol. 89, 573-579 (1975).
- Fahrbach, S. E., Meisel, R. L., Pfaff, D. W. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol. Behav. 35, 985-992 (1985).
- Morgan, M. A., Pfaff, D. W. Effects of estrogen on activity and fear-related behaviors in mice. Horm. Behav. 40, 472-482 (2001).
- Gorzek, J. F., et al. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med. Sci. Sports Exerc. 39, 248-256 (2007).
- Kadi, F., et al. The effects of physical activity and estrogen treatment on rat fast and slow skeletal muscles following ovariectomy. J. Muscle Res. Cell Motil. 23, 335-339 (2002).
- Konhilas, J. P., et al. Sex modifies exercise and cardiac adaptation in mice. Am. J. Physiol. Heart Circ. Physiol. 287, 2768-2776 (2004).
- Caligioni, C. S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 4, (2009).
- Goldman, J. M., Murr, A. S., Cooper, R. L. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B. Reprod. Toxicol. 80, 84-97 (2007).
- McLean, A. C., Valenzuela, N., Fai, S., Bennett, S. A. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp. , (2012).
- McKee, L. A., et al. Sexually dimorphic myofilament function and cardiac troponin I phosphospecies distribution in hypertrophic cardiomyopathy mice. Arch. Biochem. Biophys. 535, 39-48 (2013).
- Konhilas, J. P., et al. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ. Res. 98, 540-548 (2006).
- Perez, N. J., Chen, H., Regan, J. A., Emert, A., Constantopoulos, E., Lynn, M., Konhilas, J. P. The impact of chemically-induced ovarian failure on voluntary cage wheel exercise and cardiac adaptation in mice. Compar. Med. In press, (2013).

