Measuring Cardiac Autonomic Nervous System (ANS) Activity in Children
Özet
Measurement of autonomic nervous system activity usually confines the researcher and participant to the laboratory, which may provide an intimidating environment to children. The VU University Ambulatory Monitoring System (VU-AMS) device can record cardiac autonomic control in any setting. The VU-AMS proved very amenable to testing in children.
Abstract
The autonomic nervous system (ANS) controls mainly automatic bodily functions that are engaged in homeostasis, like heart rate, digestion, respiratory rate, salivation, perspiration and renal function. The ANS has two main branches: the sympathetic nervous system, preparing the human body for action in times of danger and stress, and the parasympathetic nervous system, which regulates the resting state of the body.
ANS activity can be measured invasively, for instance by radiotracer techniques or microelectrode recording from superficial nerves, or it can be measured non-invasively by using changes in an organ’s response as a proxy for changes in ANS activity, for instance of the sweat glands or the heart. Invasive measurements have the highest validity but are very poorly feasible in large scale samples where non-invasive measures are the preferred approach. Autonomic effects on the heart can be reliably quantified by the recording of the electrocardiogram (ECG) in combination with the impedance cardiogram (ICG), which reflects the changes in thorax impedance in response to respiration and the ejection of blood from the ventricle into the aorta. From the respiration and ECG signals, respiratory sinus arrhythmia can be extracted as a measure of cardiac parasympathetic control. From the ECG and the left ventricular ejection signals, the preejection period can be extracted as a measure of cardiac sympathetic control. ECG and ICG recording is mostly done in laboratory settings. However, having the subjects report to a laboratory greatly reduces ecological validity, is not always doable in large scale epidemiological studies, and can be intimidating for young children. An ambulatory device for ECG and ICG simultaneously resolves these three problems.
Here, we present a study design for a minimally invasive and rapid assessment of cardiac autonomic control in children, using a validated ambulatory device 1-5, the VU University Ambulatory Monitoring System (VU-AMS, Amsterdam, the Netherlands, www.vu-ams.nl).
Protocol
1. Preparation: Starting Up
- You need:
- a VU-AMS5fs ambulatory recording device (including an infrared interface cable that either connects to the RS232 serial port of a PC or to a USB port).
- 7 electrodes (we used ConMed 1690-003).
- 2 charged AA-batteries.
- an empty CompactFlash memory card (the VU-AMS5fs has been extensively tested with the 1GB 80x CF card from Transcend (TS1GCF80), but other CF cards should work too).
- a laptop or PC with flash card reader and the Data Analysis Management Software (DAMS) suite installed.
- a stopwatch.
- music player with children’s stories and headphones and a small self-inflatable air mattress are optional.
- Check the time and date settings on the laptop/PC, since these will be recorded as metadata on your files. Put the empty memory card and full batteries in the VU-AMS device (successful placement is signalled by a triple beep). When the device is on standby, the green light will flash twice every ten seconds. This indicates it is ready, but not recording. Now connect the device to the laptop using the provided cable and start up the DAMS program. Initiate communication with the device (select the tab ‘device’ and choose the appropriate connection mode, infrared cable or bluetooth).
- Have the participant take off his/her upper body wear. In the places where the electrodes will be placed,clean the skin with alcohol-wipes, and place the seven electrodes on the chest and back (Figure 1). Then attach the lead wires following the color scheme, and connect them to the device.
- Check the battery type and battery voltage indication (this should be about 3.4 V for alkaline and about 2.4 V for rechargeable NiMH batteries). Fill out the identification field. The typical sampling frequencies are as shown in the figure (Figure 2).
- Measure the distance between the two chest electrodes in millimeters, and fill this out in the field ‘ICG-V distance’. Then click ‘send settings’ to send the current settings/ID to the device.
- Now, the ‘Online’ option of the program should be used to display the ECG, ΔZ (this is the respiration) and dZ/dt (this is the ICG). The ΔZ signal reflects the base impedance across the thorax, which after appropriate filtering can be used to extract the respiration signal with high fidelity 7. The dZ/dt signal is the ΔZ differentiated over time and reflects rapid changes in ΔZ linked to the ejection of blood from the ventricle into the aorta.
- A clear QRST-complex should be detectable in the ECG. The R-wave should be upward and it should be the peak with the largest (absolute) amplitude in either direction.
- The ΔZ should be within -0.5 and +0.5 Ω most of the time and dZ/dt between -1 and +1 Ω/sec.
- Z0 should always stay within an 8 to 20 Ω range. This variation reflects the fact that the thorax impedance signal depends on the distance between the measuring electrodes which is a function of the child’s height, and the ‘wetness’ of the thorax column enclosed by the measuring electrodes, differences in body composition (e.g. BMI) can affect the amplitude of the dZ/dt signal (fat mass containing less water than muscle). Individual differences in absolute Z0 amplitude are also reflected in the ΔZ signal but this does not affect the determination of systolic time intervals, which are amplitude-independent.
- The ΔZ signal should reflect deep breathing of the subject clearly (instruct the child to take a slow deep breath and exhale slowly).
- In the ICG the typical upward waveform reflecting the cardiac ejection phase should be clearly detectable. Light movement of the subject should not distort the dZ/dt signal. If these criteria are not met, re-clean the skin and re-attach the electrodes until satisfactory signals are obtained.
- When good signals are attained, start data recording by pressing the ‘start’ button. You will hear a beep acknowledging the start of the recording and the green light will start flashing once every three seconds. The registration has now started. Close the VU-DAMS program. You can now disconnect the device from the interface cable.
2. The Registration Period
- Once the registration has started, ask the child to lie down for the first experimental condition. When the child has been in the supine position (without head-up tilt) for two minutes, you shortly (< 2 s) press the small black button on top of the device. Pressing this button marks a special event, and will later on help you identify the start of this condition in your data.
- After four minutes, press the event button again. This signals the end of the lying down condition. Now have the child sit up and repeat the procedure for this second condition. Press the button, wait four minutes and press the button again. The children are instructed to rest quietly during these conditions.
- To stop the measurement, press and hold down the button for at least 3 sec. The light will flash every 10 sec to indicate it has stopped and is in ‘stand by’ mode. Once the device has stopped, you may disconnect the lead wire plug from the connector and the lead wires from the electrodes.
- Remove the batteries and flash card form the VU-AMS device and place the flash card in the reader unit. Move the acquired files to a designated directory (typically the name of the directory will be identical to the subject identifier used in the identification field).
3. Processing the Data
- Upon opening the data with VU-DAMS program, the data will be automatically converted from raw data format (extension .5fs) to a new format (extension .amsdata). This is the data file that VU-DAMS will be using in the ensuing steps.
- First extract the Inter Beat Interval time series from the ECG signal. Select the Detect R-peaks tab. An automated algorithm will detect all R-peaks in the ECG signal and select (if present) periods with very low ECG quality for removal. In the upper left hand corner the number of Blue (correct), Yellow (medium suspicious) or RED (highly suspicious) is indicated. By pressing ‘.’ (DOT) the cursor is moved to the next suspicious R-peak and the user can delete or add markers for R-waves by hand. It is recommended that at least all highly suspicious beats are visually inspected.
- The main aim is to obtain a mean value for the heart rate, the preejection period (PEP) and measures of respiratory sinus arrhythmia (RSA, HF, RMSSD) across the experimental conditions used. Therefore proceed by indicating which periods in the raw data correspond to these conditions. This process is called ‘data labeling’. Select the Label Data tab. Two panels show the heart rate signal and movement signal respectively, as well as the actual time of the recording.
- Place the mouse cursor in the top bar where it says “click and drag to add labels” at around the start time of your first condition and drag the mouse to the end time of that condition. These times are either obtained from a written record of start and stop times (that you noted down during data collection) or you can use the start and stop markers obtained from pressing the button at the start and end of each condition, which are the vertical lines running across the HR and movement graphs.
- Each label can be given a (unique) identifier to signal a particular condition. In our case we have only one category for our labels: experimental condition. This category has two values: lying down and sitting up.
- VU-DAMS needs to be made aware of the experimental design by a so-called label configuration file (label.cfg). This is an ASCII file that can be opened with most text editors and, for example, looks like this:
# exp_condition
10 lying down
11 sitting up - By placing the label.cfg file in the directory of the .amsdata files, it will be automatically loaded by the VU-DAMS program. Once a label has been made, a pop up screen will appear with the categories/values listed in the label.cfg file. Select ‘lying down’ for the first label and ‘sitting up’ for the second label.
- After labeling, select the ‘Impedance scoring’ tab to score the PEP in the impedance cardiogram. For each of the conditions an ensemble averaged dZ/dt waveform is displayed, time-locked to the ECG R-peak. An ensemble averaged ECG is presented below the dZ/dt waveform. Place the four vertical cursors in the correct positions: ECG Q-wave onset (start of electrical activity), ICG B-point (start of the ejection phase), ICG dZ/dt-min (maximal ejection speed), and ICG X-point (aortic valve closure – end of ejection phase).
- Next, select the ‘Respiration Scoring’ tab to score the peak-valley RSA using the respiratory and ECG signals. Automated breath-to-breath scoring of the respiratory interval and the shortest interbeat interval during inspiration and the longest interbeat interval during expiration can now be inspected. Typically the automated detection algorithm should not classify more than 15% of the breaths as deviant – otherwise inspect the respiration signal and tune the parameters of the detection algorithm as needed.
Guidelines for visual inspection of the ECG, ICG and respiration signals and interactive PEP and RSA scoring can be found on the VU-AMS website, www.vu-ams.nl.
- Finally, select the Label Information tab. A table with the results appears after calculation. Each row represents the average value of a series of physiological parameters (heart rate, PEP, RSA, RR) for each labeled time period. The first column has the subject identifier (Label_ID). The last column indicates the values of all categories used during labeling (here only a single category ‘experimental condition’ with two values, ‘sitting up’ and ‘lying down’). Spectral powers of the interbeat interval times series are given only for labels with a minimum length of 4 min (otherwise the missing code is displayed). The spreadsheet in this display can be exported to ASCII or EXCEL for further statistical analyses.
Representative Results
In the Amsterdam Born Children and their Development study, a Dutch prospective, longitudinal birth cohort, the measurement protocol was started in 3,097 children 6. Approval was obtained from the Academic Medical Center Medical Ethical Committee, the VU University Medical Center Medical Ethical Committee and the Registration Committee of Amsterdam. All participating mothers gave written informed consent for themselves and their children.
As the monitors are lightweight and unobtrusive, the children tolerated these measurements very well. We do not have data on the refusal rate, but experience taught us that only a few children resisted the placement of the electrodes and thereby obstructed further assessment. Of the 3,097 registrations, 0.7% were lost due to either equipment failure or misplacement of files. Out of the 3,074 registrations left, 98.7% were of children who completed the entire protocol (n = 3,056). Within each of the labelled time periods (we originally labelled four time periods, but later summarized these to two), we encountered unclear ICG signals, meaning PEP could not be determined. This led to a loss of 1.5% in the first out of four labelled periods, 2.4% in the second, 2.8% in the third and 4.1% in the fourth period. Complete data on PEP in all time periods was available in 2,797 cases (91.5%, thus 8.5% loss due to unclear ICG signals). Complete data on heart rate (HR), pre-ejection period (PEP) and respiratory sinus arrhythmia (RSA), as well as sex and age, was available from 2,761 children; in this final step, 1.3% data loss occurred, due to unknown reasons. Overall, 89.2% of the started registrations led to full subject data. The mean age of the children was 5.7 years (SD 0.5; interquartile range 5.0:6.5), and their BMI was 15.5 kg/m2 (SD 1.5; interquartile range 13.9:17.2).
The mean values of the major outcome variables HR, PEP, and RSA are given in Table 1 and graphically depicted in Figure 3, separately for boys and girls. HR (both lying down and sitting up) and PEP (only sitting up) were higher in girls than in boys (both postures). RSA was lower in girls than in boys (both postures). The higher values for HR in girls are likely to be caused by the lower vagal (parasympathetic) cardiac control. Their sympathetic cardiac control was not different or even lower than that in boys (sitting up).
In both sexes, HR was higher when sitting up compared to lying down, whereas RSA was lower when sitting up. This reflects the lower vagal control when sitting up. PEP was shorter lying down then sitting up. This effect was also as expected, and it reflects the outcome of opposite processes: lower sympathetic activity (lengthens PEP) while lying down with increased preload (shortens PEP)7.
| Boys | Girls | ||||||||||
| Lying down | Sitting up | Lying down | Sitting up | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Heart rate (bpm) | 83.9 | 9.5 | * | 89.1 | 10 | *† | 86.9 | 10.1 | 92.4 | 10.4 | † |
| Pre-ejection period (msec) | 76.9 | 11.8 | 78.5 | 12.2 | *† | 77.7 | 10.3 | 81 | 11.7 | † | |
| Respiratory sinus arrythmia (msec) | 127.0 | 60.4 | * | 115.7 | 55.8 | *† | 121.7 | 56.8 | 108.7 | 51.9 | † |
Table 1. Cardiac autonomic nervous system measures in boys and girls, by posture on posture difference. p < 0.05 for one sample T-test on sex difference. † p < 0.05 for paired samples T-test.
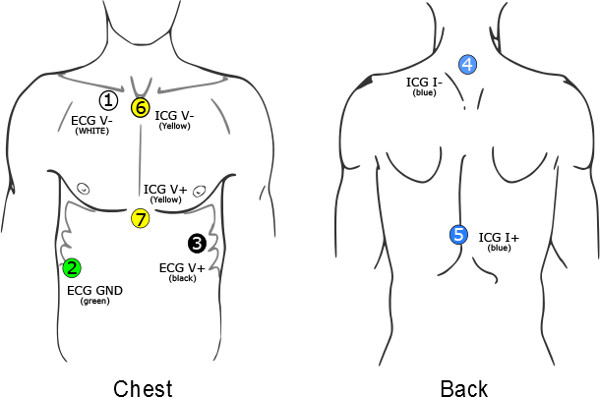
Figure 1. The seven electrodes should be placed on the participant’s chest and back. The first ECG electrode (V-) is placed slightly below the right collar bone 4 cm to the right of the sternum. The second ECG electrode (V+) is placed at the apex of the heart over the ninth rib on the left lateral margin of the chest approximately at the level of the processus xiphodius. The third ECG electrode (GND) is a ground electrode and is placed on the right side, between the lower two ribs at the right abdomen. The first ICG measuring electrode (V1) is placed at the top end of the sternum, between the tips of the collar bones. The second ICG measuring electrode is placed at the xiphoid complex of the sternum, where the ribs meet. The two current electrodes are placed on the back: I- on the spine over the cervical vertebra C4, at least 3 cm (1 in) above the ICG measuring elec-trode V-, and I+ between thoracic vertebrae T8 and T9 on the spine, at least 3 cm (1″) below the ICG measuring elec-trode V2. The ICG electrode placement takes into account that the largest part of the left ventricle driven change in thorax impedance is captured by the column between the suprasternal notch and the processus xiphoideus.
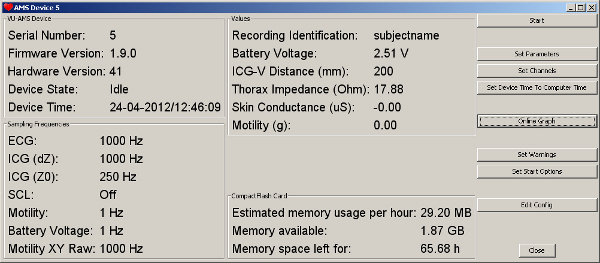
Figure 2. The typical settings used for a recording as displayed by the DAMS software after connecting to the VU-AMS5fs device. Click here to view larger figure.
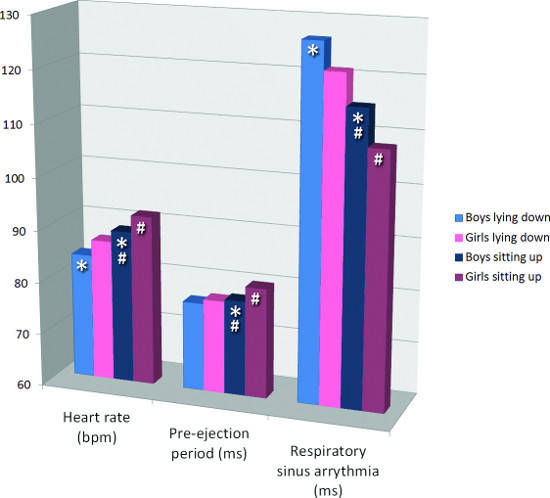
Figure 3. Cardiac autonomic nervous system measures in boys and girls, by posture. * indicates p < 0.05 for one sample T-test on sex difference. # indicates p < 0.05 for paired samples T-test on posture difference.
Discussion
We used an ambulatory recording device to measure cardiac autonomic control in 3097 children, aged between 5 and 7 years. Seven electrodes sufficed to measure the ECG and ICG from which the heart rate, heart rate variability and the systolic time intervals were extracted. Heart rate variability in the respiratory frequency band (RSA) is a valid indicator of cardiac parasympathetic activity. The systolic time interval, PEP, by reflecting cardiac contractility, is a valid indicator of cardiac sympathetic activity. The mean values obtained for HR, PEP and RSA, the effects of posture changes and the differences between boys and girls were in line with what would be expected from the literature.
As ambulatory monitoring removed the necessity of assessment in a laboratory our recordings could be done in various locations (e.g. school, sports center, science museum) without differences in signal recording quality. However, it is of crucial importance to standardize within or between subject comparisons for posture and physical load, as afterload and preload effects can co-determine the PEP without any changes in cardiac sympathetic drive 7. We conclude that ambulatory recording of the ECG and ICG in large samples of children is highly feasible and propose the current standardized study design as a useful template for future assessments of cardiac autonomic control in children.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
AEvD was supported by the Dutch Heart Foundation (DHF-2007B103). The authors would like to thank all mothers and children in the Amsterdam Born Children and their Development (ABCD) study, and the entire development and maintenance team of the VU-AMS system at the Division for Instrumentation – the Department of Psychophysiology (VU University, Amsterdam, the Netherlands).
Materials
| Name of Reagent/Material | Company | Catalogue Number | Yorumlar |
| VU-AMS5fs ambulatory recording device & infrared interface cable | VU University Amsterdam | n/a | http://www.vu-ams.nl |
| Electrodes | ConMed | 1690-003 | |
| AA-batteries | |||
| CompactFlash memory card | |||
| Laptop/pc with flash card reader | |||
| VU-DAMS software suite | VU University Amsterdam | free download, http://www.vu-ams.nl | |
| Stopwatch | |||
| Music player & headphones | optional | ||
| Self-inflatable air mattress | optional |
Referanslar
- de Geus, E. J., Willemsen, G. H., Klaver, C. H., van Doornen, L. J. Ambulatory measurement of respiratory sinus arrhythmia and respiration rate. Biol. Psychol. 41, 205-227 (1995).
- Goedhart, A. D., van der, S. S., Houtveen, J. H., Willemsen, G., de Geus, E. J. Comparison of time and frequency domain measures of RSA in ambulatory recordings. Psychophysiology. 44, 203-215 (2007).
- Goedhart, A. D., Kupper, N., Willemsen, G., Boomsma, D. I., de Geus, E. J. Temporal stability of ambulatory stroke volume and cardiac output measured by impedance cardiography. Biol. Psychol. 72, 110-117 (2006).
- Riese, H., Groot, P. F. C., van den Berg, M., et al. Large-scale ensemble averaging of ambulatory impedance cardiograms. Behavior Research Methods Instruments & Computers. 35, 467-477 (2003).
- Willemsen, G. H., de Geus, E. J., Klaver, C. H., van Doornen, L. J., Carroll, D. Ambulatory monitoring of the impedance cardiogram. Psychophysiology. 33, 184-193 (1996).
- van Dijk, A. E., van Eijsden, M., Stronks, K., Gemke, R. J., Vrijkotte, T. G. Prenatal stress and balance of the child’s cardiac autonomic nervous system at age 5-6 years. PLoS ONE. 7, e30413 (2012).
- Houtveen, J. H., de Groot, P. F., de Geus, E. J. Effects of variation in posture and respiration on RSA and pre-ejection period. Psychophysiology. 42, 713-719 (2005).
- Goedhart, A. D., Willemsen, G., Houtveen, J. H., Boomsma, D. I., De Geus, E. J. Comparing low frequency heart rate variability and preejection period: two sides of a different coin. Psychophysiology. 45, 1086-1090 (2008).
- van Dijk, A. E., van Eijsden, M., Stronks, K., Gemke, R. J., Vrijkotte, T. G. Cardio-metabolic risk in 5-year-old children prenatally exposed to maternal psychosocial stress: the ABCD study. BMC Public Health. 10, 251 (2010).
- van Lien, R., Goedhart, A., Kupper, N., Boomsma, D., Willemsen, G., de Geus, E. J. Underestimation of cardiac vagal control in regular exercisers by 24-hour heart rate variability recordings. Int. J. Psychophysiol. 81, 169-176 (2011).

