Özet
Eye tracking has long been used to study gaze patterns in typically-developing individuals, but recent technological advancements have made its use with clinical populations, including autism, more feasible. While eye-tracking young children with autism can offer insight into early symptom manifestations, it involves methodological challenges. Suggestions for best practices are provided.
Abstract
The rise of accessible commercial eye-tracking systems has fueled a rapid increase in their use in psychological and psychiatric research. By providing a direct, detailed and objective measure of gaze behavior, eye-tracking has become a valuable tool for examining abnormal perceptual strategies in clinical populations and has been used to identify disorder-specific characteristics1, promote early identification2, and inform treatment3. In particular, investigators of autism spectrum disorders (ASD) have benefited from integrating eye-tracking into their research paradigms4-7. Eye-tracking has largely been used in these studies to reveal mechanisms underlying impaired task performance8 and abnormal brain functioning9, particularly during the processing of social information1,10-11. While older children and adults with ASD comprise the preponderance of research in this area, eye-tracking may be especially useful for studying young children with the disorder as it offers a non-invasive tool for assessing and quantifying early-emerging developmental abnormalities2,12-13. Implementing eye-tracking with young children with ASD, however, is associated with a number of unique challenges, including issues with compliant behavior resulting from specific task demands and disorder-related psychosocial considerations. In this protocol, we detail methodological considerations for optimizing research design, data acquisition and psychometric analysis while eye-tracking young children with ASD. The provided recommendations are also designed to be more broadly applicable for eye-tracking children with other developmental disabilities. By offering guidelines for best practices in these areas based upon lessons derived from our own work, we hope to help other investigators make sound research design and analysis choices while avoiding common pitfalls that can compromise data acquisition while eye-tracking young children with ASD or other developmental difficulties.
Protocol
1. Eye-Tracking Equipment
Although a variety of eye-tracking systems are commercially available, those that are most conducive to testing young children with ASD share the following features:
- First and foremost, the eye-tracker needs to account for head motion, which if uncorrected, can compromise the integrity of acquired gaze data. While many older systems ensured accurate tracking through head stabilization via the use of a chin-rest or head mounted systems (see Figure 1), these options are not ideal eye-tracking solutions for young children who may resist efforts to restrict head movement, or have equipment placed upon them. Fortunately, most modern commercial infrared video eye-tracking systems use a reference system based on corneal reflections that are resilient to minor head movements. Eye-tracking systems that either offer integrated or remote head tracking solutions are preferred and are now widely available.
- Unobtrusive eye-tracking systems that do not interfere with the testing session are recommended for testing children with ASD. These can be models that are integrated within a display monitor (e.g., Tobii Technology models TX300, T60XL, or T120; SensoMotoric Instruments model RED500), or table-top versions (e.g., Tobii Technology TX300, X120; Applied Science Laboratories model D6 Optics; SR Research model EyeLink 1000) placed inconspicuously within range of the participant. As table-top versions are not locked into a specific screen size, they offer increased methodological flexibility but are less automated and may require more manual adjustment.
- Researchers should also select an eye-tracking system with a sampling rate appropriate for addressing their research questions. Most corneal reflection eye-tracking systems have a minimum sampling rate of 50 Hz (i.e., 50 data points per second), which is adequate for examining young children’s perceptual patterns during their visual scanning of static images13 and dynamic videos10. However, researchers interested in subtle oculomotor behavior14 (e.g., smooth pursuit, gain, and/or express saccades) will want to invest in a system with a higher sampling rate (i.e., ≥ 250 Hz). Be aware that in some systems that allow for several sampling rate options, higher sampling rates often are enabled at the expense of freedom of head movement, making it more difficult to keep a kinetic child within eye-tracking range. Higher frame rates require faster sampling and processing of video data, which is typically accomplished by cropping the image obtained from the camera. This results in a reduction of the used field of view, which thereby reduces the range of permissible head movement. Researchers who use systems that offer different sampling rate options should select one that is high enough to address their research question but low enough to allow for expected levels of head movement.
2. Testing Environment and Stimuli
- Sparse room décor is recommended for the immediate eye-tracking environment in order to minimize the chances that the child’s attention is drawn outside the display. Similarly, a dimly lit room helps reduce the salience of competing non-display stimuli.
- However, because some young children with ASD may experience visual and/or auditory hypersensitivities, researchers should avoid testing in a completely darkened room that heightens the brightness of the display or including overly loud or jarring sound effects in their presentations, as these may be aversive for some children with ASD and result in reduced testing compliance. Darkened environments may also increase pupil dilation which can make the pupil more difficult to track, though this may vary depending on the eye-tracking equipment used. For most cases, standard office lighting is recommended.
- To further reduce the chances of the child being distracted away from the display, the experimenter should not be visible to the participating child. This may be achieved by placing a partition between the eye-tracking station and the experimenter-manned host computer or by simply positioning the experimenter out of view from the participant. A second camera can help the experimenter retain a view of the participant in this situation. Indeed, some commercial eye-trackers integrate a camera within the display so that video of the participant is relayed in real time to the host computer for experimenter monitoring.
3. Procedures
- Young children, particularly those with an ASD, may be apprehensive about experiencing a novel testing environment. While prior familiarity with the testing space and/or experimenter may help to mollify these feelings, this is not always possible. At a minimum, apprehensive children should be accompanied by a parent or familiar adult throughout the testing session. In some cases, researchers should be prepared to be patient while the child becomes accommodated to the new environment.
- Before the testing session begins, the experimenter may choose to have a children’s video or cartoon playing on the display monitor. This often helps make the child feel more at ease while also ensuring attention is directed towards the testing display. The experimenter may then take advantage of the child’s captured attention to position the child within eye-tracking range and transition directly into the calibration sequence.
- Seating for the child must allow for vertical adjustment in order to ensure that all children, regardless of height and posture, can be positioned within eye-tracking range. While the distance of the chair from the display will depend upon the size of the screen and the desired visual angle, the height of the chair needs to be adjusted based upon the child’s stature so that line of sight is standardized across all participants. Because not all children sit in the chair in exactly the same way, it is also often necessary to slightly adjust the participant-to-display distance in order to ensure that each child is positioned at the optimal distance from the screen for acquiring high quality data. This distance will be specified by the eye-tracking manufacturer, and is most easily achieved by using a mobile chair.
- Experimenters may first attempt to have the child, if small enough, sit alone in a buckled car seat on an adjustable lift or in a portable high chair that attaches to adjustable-height office chair. We have also had success using a Rifton chair, particularly with children who have used it previously in classroom or home settings, as it is easy to position and helps restrict mobility that may result in data loss.
- Children who only remain compliant while sitting upon the lap of the caregiver, or who require the body support the caregiver can provide, can also be accommodated by having caregiver sit in an office chair that may be raised or lowered to position the child within the standardized distance parameters. See Figure 2 for an example of this set-up. Statistics regarding lap versus chair sitting should always be retailed for analyzing possible confounds. In order to ensure that only the child’s eyes are acquired by the eye tracker, researchers may have caregivers wear infrared-blocking or opaque sunglasses, or simply instruct them to close their eyes during testing. Caregivers should also be instructed to be still and refrain from verbally or non-verbally communicating with the child during the testing procedure.
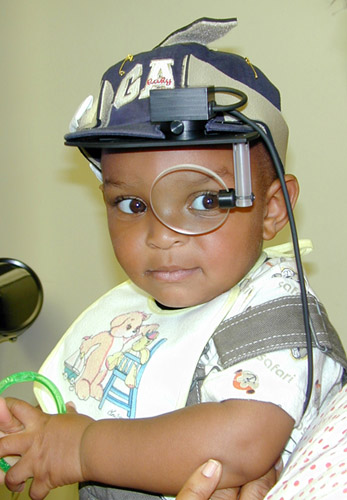
Figure 1. A head-mounted eye-tracking system.

Figure 2. A child may sit on the lap of the caregiver if the child requires physical assistance in sitting, or if it is necessary for maintaining compliance.
- For eye-tracking systems that provide the experimenter with a window displaying the participant’s eyes within the range of permitted head movement, the child’s eyes should be positioned in the middle of the window in order to increase the chances that the eye tracker will retain an image of the eye even if the child slouches, straightens or sways during testing.
- Once properly positioned, the experimenter should begin the calibration procedure. As young children with ASD may be unable or unwilling to follow verbal instructions to look to specific locations on the screen (as is typical for many calibration sequences), the use of dynamic stimuli, accompanied by sound, may more effectively capture attention and thus result in more accurate gaze data. Typically, a 5-point sequence is brief enough to retain the child’s attention while also providing an accurate calibration. Eye-tracking studies with infants often employ just a 2-point calibration while a 9-point calibration is typical for investigations with adolescents and adults.
- Experimenters may maximize the child’s visual attention to the display during testing by designing a concise and compelling task that has minimal task demands (e.g., a passive viewing task). Further, including an inter-stimulus animation with an accompanying sound effect (perhaps similar to those used during the calibration sequence) can help redirect attention to the display for children whose attention has lapsed. Additionally, positioning this inter-stimulus animation in a predefined location can ensure that all visual scanning patterns begin in the same spot for all participants.
- If the research task is lengthy, the experimenter may use this inter-stimulus animation as an “anchor” to determine if calibration drift is occurring. Typically, if drift exceeds 3 degrees of visual angle, re-calibration should be administered. Additionally, if multiple tasks or trials are included, re-calibration is recommended between each one to eliminate drift over the course of testing.
4. Analysis
- Most eye-tracking systems yield raw data files that include, at a minimum, a timestamp, X and Y coordinates of the point of regard (sometimes for both eyes), the distance from the display or stimulus, along with an index that characterizes an event or change in stimulus presentation. Some software programs also yield information about pupil diameter and fixation metrics.
- How one chooses to condense the vast amount of raw data is determined by the research question. Most often the goal is to characterize metrics of fixation density and/or oculomotor dynamics. However, once these constructs are characterized, superordinate constructs such as attention and memory can be examined under specific design conditions.
- Fixation Density: While many different algorithms exist that characterize fixation density15, all analyze two primary components: temporal and spatial information. For example, a fixation can be defined as the point of regard remaining within a diameter of 1° of visual angle for at least 100 milliseconds, though these parameters are often anchored by the research question. Common dependent variables include number of fixations, average duration of fixation, and total fixation time, and the spatial arrangement and/or sequence of individual fixations (i.e., scan paths)16.
- Fixation analyses are often conducted within predefined “Areas of Interest” (AOI). Researchers may be interested in whether children with and without ASD differ in their fixation time to specific AOIs (such as the eyes on a face), their latency to first fixate AOIs, or in the patterns of their gaze shifts between AOIs. Additionally, the metrics listed in 4.3 can also be applied to AOI analyses.
- Children with ASD, particularly those with impairments in sustaining attention, often exhibit more missing gaze data than controls. This may occur due less visual attention to onscreen stimuli or by excessive blinking (sometimes produced by an overly bright display or a too dark testing environment). To control for missing data differences between groups, researchers may want to conduct analysis as a proportion of gaze time onscreen instead of in absolute values that can be confounded by missing data. Further, in order to protect against unreliable data caused by insufficient sampling, researchers should require that all participants who are included in the final sample pass a “minimum time” cut off. The specificity of this cut off will vary by study, but in general participants with more missing than recorded data should be considered suspect.
- In contrast to fixation analysis, velocity-based algorithms incorporate the change in Euclidean distance between subsequent recordings and focus primarily on saccades. A saccade is indicated when the velocity (distance/time) exceeds a certain threshold. If concurrent recordings do not exceed this velocity threshold for a specified period of time, a fixation is indicated.
- Oculomotor Dynamics: Characterizing oculomotor dynamics requires a high sampling eye-tracking system that is sufficiently sensitive to subtle changes in eye position and eye movement. Although many dependent variables can be investigated within the scope of oculomotor dynamics including saccades, ocular drift, and pursuit, all indices rest upon the velocity of eye movement. Characterizing this velocity is based on two properties (i.e., distance and time) and thus allows for the examination of other properties of oculomotor dynamics, including the distribution or pattern of saccade velocities, the distribution or pattern of saccade amplitude, the distribution or pattern of saccade duration, as well as the latency of saccade and accuracy of saccade termination (i.e., gain). Common paradigms include visually guided saccade tasks, antisaccade tasks, memory guided saccade tasks, and predictive saccade tasks.7, 17 The extant literature contains a large body of research on saccade dynamics that could benefit interested researchers18-20.
5. Representative Results
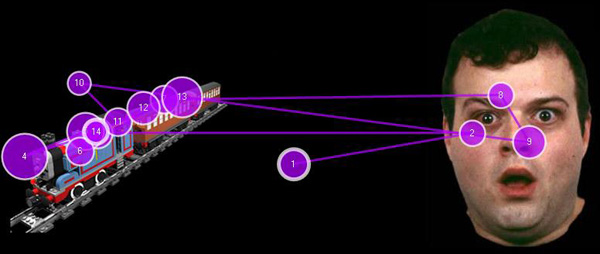
Figure 3. Figure 3 represents a fixation map generated in research by our group. Shown here are the individual fixations, depicted by purple circles, by a single child with ASD while viewing a static image. Fixations from this and similar tasks are analyzed across participants to determine if children with and without ASD differ in their visual attention to various AOIs.

Figure 4. Figure 4 represents an example of a final product that includes many of the steps described above, including: 1) applying a fixation filter to raw gaze data, 2) assigning fixations to specific AOIs, and 3) condensing and comparing patterns of fixations across a group of typically developing (TD) children and a group of children with ASD. More specifically, this figure demonstrates that within a visual exploration paradigm, children with ASD explore (i.e., fixate) fewer social images TD children when “High Autism Interest” (HAI) objects are concurrently displayed. When “Low Autism Interest” (LAI) objects are presented with social stimuli, however, exploration of social images does not differ significantly between groups, suggesting that social attention in ASD is modulated based upon the relative salience of competing stimuli.21
Discussion
The emergence of eye-tracking as an objective and accessible tool for examining perceptual characteristics of psychiatric disorders has facilitated research into the abnormal visual attention and oculomotor patterns that contribute to clinical characteristics of ASD. A particularly promising application of this work has been to study young children with ASD in order to capture early-emerging developmental mechanisms of the disorder. While investigations with this age group are critical for illuminating the early course and characteristics of ASD, doing so with traditional behavioral paradigms has often been challenging given the social and communicative impairments that exist in this population. Eye-tracking can alleviate some of these task demands by providing a direct and quantifiable measure of visual preferences and gaze behavior. Ultimately, this approach may help reveal important information during a critical period in the development of ASD, a process that in turn could inform early identification and intervention efforts.
Despite these benefits, eye-tracking young children with ASD is complicated by a number of methodological challenges. The current protocol details guidelines for implementing eye-tracking with this population. While the suggestions outlined here are limited to common and commercially available corneal-reflection eye-tracking systems, particularly those that do not restrict participant movement and can correct for head motion, they are designed to provide general instruction for interested researchers. Optimal design is often study specific, but general recommendations include selecting equipment and creating a testing environment that are conducive for studying this population, implementing procedures that minimize obstacles and potential distractions, and pursuing appropriate analytic strategies.
The adoption of such practices may expedite eye-tracking research aimed at examining early symptom manifestations of ASD, but they may also pertain to research groups interested in eye-tracking young children more broadly, including infants22, as well as children with developmental disabilities other than autism. Further, researchers may want to build upon these recommendations by incorporating eye-tracking with other physiological measures, such as EEG, galvanic skin conductance and heart-rate monitoring, to provide a more comprehensive profile of psychobiological response. The increasing availability of mobile eye-tracking systems also enhances the possibility to extend the study of gaze behavior in ASD into more ecologically-valid contexts, including live social interaction. These innovative designs are expected to rise in popularity in the coming years.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
We thank all the children and caregivers who participated in our eye-tracking studies at the Callier Center for Communication Disorders at the University of Texas at Dallas, and at the UNC Carolina Institute for Developmental Disabilities.
N. Sasson was supported by Grant Number UL1RR024982, titled, “North and Central Texas Clinical and Translational Science Initiative” (Milton Packer, M.D., PI) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
J. Elison was supported by an NRSA award (5-T32-HD007376) from NICHD to the Carolina Consortium on Human Development at the Center for Developmental Science, UNC.
Materials
| Material Name | Tip | Company | Catalogue Number | Comment |
| Eye-tracker | A stand-alone corneal reflection based system. Eye-tracker is integrated into 24″ TFT widescreen monitor. Records at 60Hz. | Tobii | Tobii 60XL | Shown in Figure 2. This is one of several systems that allows for head motion (in this case, within a cubic space of 40x20x27cm from a distance of 60cm, while retaining an average accuracy of ~0.5° of visual angle). |
| Eye-tracker Control Software | Tobii | Tobii Studio v. 2.1.14 | ||
| Fixation Analysis Program | Tobii | Tobii Studio v. 2.1.14 | Fixation analysis in Figure 3 generated using this program. Output in Figure 4 generated in external software. |
Referanslar
- Sasson, N. J., Tsuchiya, N., Hurley, R., Couture, S. M., Penn, D. L., Adolphs, R., Piven, J. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia. 45, 2580-2588 (2007).
- Pierce, K., Conant, D., Hazin, R., Stoner, R., Desmond, J. Preference for geometric patterns early in life as a risk factor for autism. Arch. Gen. Psychiat. 68, 101-109 (2011).
- Combs, D. R., Chapman, D., Waguspack, J., Basso, M. R., Penn, D. L. Attention shaping as a means to improve emotion perception deficits in outpatients with schizophrenia and impaired controls. Schizophr. Res. 127, 151-156 (2011).
- Ames, C., Fletcher-Watson, S. A review of methods in the study of attention in autism. Dev Rev. 30, 52-73 (2010).
- Boraston, Z., Blakemore, S. J. The application of eyetracking technology in the study of autism. The Journal of Physiol. 581, 893-898 (2007).
- Simmons, D. R., Robertson, A. E., McKay, L. S., Toal, E., McAleer, P., Pollick, F. E. Vision in autism spectrum disorders. Vision Res. 49, 2705-2739 (2009).
- Karatekin, C. Eye tracking studies of normative and atypical development. Dev Rev. 27, 283-348 (2007).
- Spezio, M. L., Adolphs, R., Hurley, R. S., Piven, J. Analysis of face gaze in autism using "Bubbles. Neuropsychologia. 45, 144-151 (2007).
- Dalton, K. M., Nacewicz, B. M., Johnstone, T., Schaefer, H. S., Gernsbacher, M. A., Goldsmith, H. H., Alexander, A. L., Davidson, R. J. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 8, 519-526 (2005).
- Klin, A., Lin, D. J., Gorrindo, P., Ramsay, G., Jones, W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 459, 257-261 (2009).
- Pelphrey, K. A., Sasson, N. J., Reznick, J. S., Paul, G., Goldman, B. N., Piven, J. Visual scanning of faces in adults with autism. J. Autism Dev. Disord. 32, 249-261 (2002).
- Chawarska, K., Shic, F. Looking but not seeing: atypical visual scanning and recognition of faces in 2 and 4-year-old children with autism spectrum disorder. J. Autism Dev. Disord. 39, 1663-1672 (2009).
- Sasson, N. J., Elison, J. T., Turner-Brown, L. M., Dichter, G. S., Bodfish, J. W. Brief report: circumscribed attention in young children with autism. J. Autism Dev. Disord. 41, 242-247 (2011).
- D’Cruz, A. K., Mosconi, M. W., Steele, S., Rubin, L. H., Beatriz, L., Minshew, N., Sweeney, J. A. Lateralized response timing deficits in autism. Biol. Psychiatry. 66, 393-397 (2009).
- Shic, F., Chawarska, K., Scassellati, B. The incomplete fixation measure. , 111-114 (2008).
- Jacob, R. J. K., Karn, K. S., Hyona, J., Radach, R., Deubel, H. Eye tracking in human-computer interaction in usability research: ready to deliver the promises (section commentary). The Mind’s Eye: Cognitive and Applied Aspects of Eye Movement Research. , 573-605 (2003).
- Sweeney, J. A., Takarae, Y., Macmillan, C., Luna, B., Minshew, N. J. Eye movements in neurodevelopmental disorders. Curr. Opin. Neurol. 17, 37-42 (2004).
- Duchowski, A. T. . Eye tracking methodology: theory and practice. , (2003).
- Goldberg, J. H., Kotval, X. P. Computer interface evaluation using eye movements: methods and constructs. Int. J. Indus. Ergonomics. 24, 631-645 (1999).
- Salvucci, D. D., Goldber, J. H. Identifying fixations and saccades in eye-tracking protocols. , 71-78 (2000).
- Sasson, N. J., Turner-Brown, L. M., Holtzclaw, T. N., Lam, K. S. L., Bodfish, J. W. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism. Research. 1, 31-42 (2008).
- Gredebäck, G., Johnson, S., von Hofsten, C. Eye tracking in infancy research Developmental Neuropsychology. 35, 1-19 (2009).

