High-throughput Saccharification Assay for Lignocellulosic Materials
Özet
A simple, rapid method for determining the saccharification potential of large numbers of plant biomass samples is described. The automated platform for this analysis involves the preparation of the plant biomass for analysis in 96 well plates and the subsequent performance of pretreatment, hydrolysis and quantification of the sugars released.
Abstract
Polysaccharides that make up plant lignocellulosic biomass can be broken down to produce a range of sugars that subsequently can be used in establishing a biorefinery. These raw materials would constitute a new industrial platform, which is both sustainable and carbon neutral, to replace the current dependency on fossil fuel. The recalcitrance to deconstruction observed in lignocellulosic materials is produced by several intrinsic properties of plant cell walls. Crystalline cellulose is embedded in matrix polysaccharides such as xylans and arabinoxylans, and the whole structure is encased by the phenolic polymer lignin, that is also difficult to digest 1. In order to improve the digestibility of plant materials we need to discover the main bottlenecks for the saccharification of cell walls and also screen mutant and breeding populations to evaluate the variability in saccharification 2. These tasks require a high throughput approach and here we present an analytical platform that can perform saccharification analysis in a 96-well plate format. This platform has been developed to allow the screening of lignocellulose digestibility of large populations from varied plant species. We have scaled down the reaction volumes for gentle pretreatment, partial enzymatic hydrolysis and sugar determination, to allow large numbers to be assessed rapidly in an automated system.
This automated platform works with milligram amounts of biomass, performing ball milling under controlled conditions to reduce the plant materials to a standardised particle size in a reproducible manner. Once the samples are ground, the automated formatting robot dispenses specified and recorded amounts of material into the corresponding wells of 96 deep well plate (Figure 1). Normally, we dispense the same material into 4 wells to have 4 replicates for analysis. Once the plates are filled with the plant material in the desired layout, they are manually moved to a liquid handling station (Figure 2). In this station the samples are subjected to a mild pretreatment with either dilute acid or alkaline and incubated at temperatures of up to 90°C. The pretreatment solution is subsequently removed and the samples are rinsed with buffer to return them to a suitable pH for hydrolysis. The samples are then incubated with an enzyme mixture for a variable length of time at 50°C. An aliquot is taken from the hydrolyzate and the reducing sugars are automatically determined by the MBTH colorimetric method.
Protocol
1. Preparation of the samples
We normally work with stems from either herbaceous or woody plants. In the case of herbaceous materials we grow the plants to maturity, and after seed development we collect dry stems once senescence is complete. The stems are chopped into 4 mm segments and placed in 2 ml vials with three milling balls. In the case of woody materials, the samples are ground to coarse sawdust with a course wood file and then placed in a ball mill for further processing.
The samples are placed in a rack within the grinding/formatting station (Figure 1). This station sequentially grinds, mixes, and weighs each sample. The number of repetitions needed per sample is established by testing the ground material in a series of preliminary experiments where the intrinsic variability for a certain particle size is determined. The 2 mL vials are pierced at the bottom and the materials are dispensed by vibration of the vial over the selected well. The vibrating arm is controlled by a feedback from the balance allowing for the accurate dispensing of the powder. The robot dispenses 4 mg/well in a standard analysis and 4 repetitions are needed in most the cases. This allows 20 plant samples/plate to be analysed.
2. Pretreatment
Once the samples are formatted, the 96-well plates are processed by an automated liquid handling system (Figure 2). Here a mild pretreatment is performed on the plant materials by adding 350 μL of acid or alkaline solutions and heating the plate on an adapted block. To avoid evaporation during the analysis, the 96 well plates are sealed with a silicone matt. The temperature and time of the pretreatment can be modified according to the materials being studied. The maximum temperature, however, is 100 °C. An alternative procedure for testing harsher pretreatments is to pretreat the materials off line and eliminate the pretreatmet from the process.
3. Hydrolysis
- After the materials are pretreated, the robot automatically removes the pretreatment solution, and washes, all the wells, 5 times with 850 μL 25 mM Acetate Buffer. The rinses are carried out by adding buffer to the pretreatment solution, and subsequently removing 50% of the total volume after allowing the solids in the sample to settle. The height of the aspiration is set at half of the liquid mass to avoid aspirating solids from the bottom of the well; there is negligible loss of solids using this approach. After these washes the pH in the well is 4.5 and the material is ready for enzymatic hydrolysis.
- An enzyme mixture containing a solution of cellulases and hemicellulases is added by the robot. Into each well, 850 μL of enzyme mixture is dispensed and the plate is moved into a 50 °C shaking incubator. The standard hydrolysis is performed for 8 h, but this time can be adapted to the purpose of the experiment. The standard enzyme loading used for grasses is 7 FPU/g of material.
4. Detection of reducing sugars
Determination of reducing sugars released after hydrolysis was performed using a modified 3-methyl-2-benzothiazolinone hydrozone (MTBH) method3. MBTH was selected as the most suitable method because it was the easiest to automate and the least susceptible to interference from compounds such as proteins. We modified this highly sensitive method for use on the robotic platform so that it could accurately quantify sugars at the concentrations present in the biomass hydrolysates, with a final volume of 250 μL which is suitable for a standard optical 96 well plate. All the steps below are performed automatically and three independent determinations of the reducing sugars were carried out for each saccharification reaction.
- 30 μL of hydrolyzate was taken from the deep well plate and mixed with 45 μL of 25 mM Sodium Acetate buffer in a skirted PCR 96 well plate.
- 25 μL of 1N NaOH and 50 μl of a solution containing 0.43 mg/ml MBTH and 0.14 mg/ml DTT were added.
- After mixing, the PCR plate was heated at 60°C for 20 min in a thermocycler or similar device that delivers exactly the same amount of heat to all 96 wells.
- The resulting reaction was transferred to an optical plate.
- Add 100 μl of oxidising reagent (0.2% FeNH4(SO4)2, 0.2% sulfamic acid and 0.1% HCl). Mix well and leave to develop for at least 1 h.
- Each plate should also contain standard reactions of 50 nmol, 100 nmol and 150 nmol glucose. The optical plates are read at 620 nm.
5. Representative Results:
Examples of different types of analyses are presented in figure 3 and 4. All the results were obtained by using the automated platform. Figure 3 represents the increase in reducing sugars released by 8 h incubations from ground poplar with increasing amounts of cellulolytic enzymes. Figure 4 presents the effects of acid and alkaline pretreatment on poplar samples. The NaOH pretreatment is more efficient that the diluted H2SO4 pretreatment in facilitating the release of sugars during hydrolysis. The amount of sugars measured after hydrolysis decreases when the pretreatment is performed using NaOH concentrations higher than 1M.
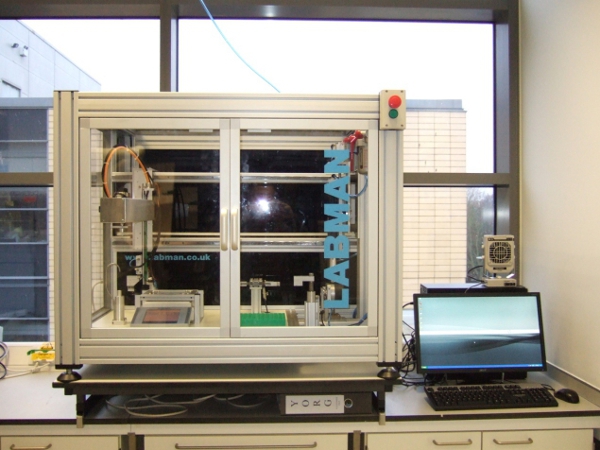
Figure 1. Robotic station for grinding and 96 well formatting of biomass

Figure 2. Liquid handling station for the High Throughput saccharification analysis
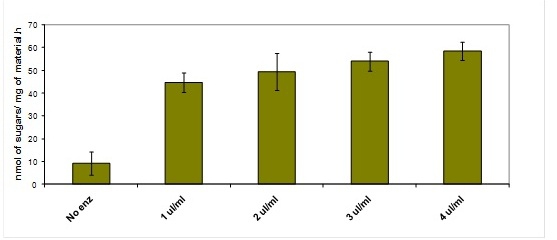
Figure 3. Effect of different enzyme loadings on the release of reducing sugar equivalents from poplar samples
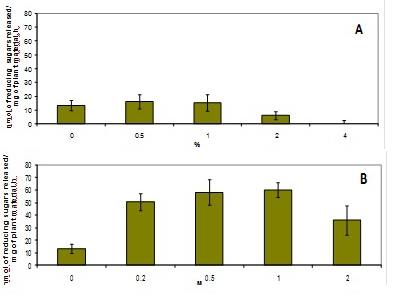
Figure 4. Effect of different pretreatments on the saccharification of poplar samples. A. Sugars released after pretreament with different percentages of H2SO4 at 90°C for 30 minutes. B. Sugars released after NaOH pretreatments at the same temperature and time than the acid pretreatment.
Discussion
Variations of the standard saccharification protocol can be used in the same platform for determining the activity of cellulolytic enzymes (i.e. by variation of the enzyme concentrations used in a plate as well as using paper as substrate); comparison of the efficiency of several enzyme mixtures on a specific material; time course for saccharification; etc.
A standard saccharification protocol to compare the saccharification potential in different plant materials involves an eight hour hydrolysis (Figures 3 and 4). Most of the plant materials analysed requires four replicates. Under these conditions, the platform can process 80 samples/day. This analysis is being used to screen large populations of barley, maize, and brachypodium in order to establish variability in saccharification potential and the genes involved in its determination4.
Açıklamalar
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank A Viksø-Nielsen (Novozymes) for the gift of the cellulolytic enzymes. This work was funded by FP7 RENEWAL and by BBSRC projects BB/G016178 and BB/G016194.
Materials
| Name of the reagent | Company | Catalogue number |
|---|---|---|
| Grinding & weighing robot | Labman Automation | |
| 2 ml micro tube with caps | Sarstedt Ltd | 72.694 |
| 5 mm stainless steel beads | Qiagen Ltd | 69989 |
| 1.2ml Abgene square well storage plates | Fisher Scientific Ltd | TUL-050-050C |
| Whatman cap mat for 96 square well plates | Fisher Scientific Ltd | 7704-0104 |
| Liquid hanling robot | Tecan Group Ltd. | Freedom Evo 200 |
| Sulphuric acid | Fisher Scientific Ltd | S/9231/PB17 |
| Sodium hydroxide | Fisher Scientific Ltd | BPE359-500 |
| Sodium acetate | Sigma-Aldrich | S8750-500G |
| Acetic acid | Fisher Scientific Ltd | A/0420/PB17 |
| Novozyme 188 | Novozyme | DCN00214 |
| Celluclast 1.5L | Novozyme | CCN03122 |
| 96 well PCR full skirt plates | Sarstedt Ltd | 72.1980.202 |
| D-Glucose | Fisher Scientific Ltd | G/0450/53 |
| 3-Methyl-2-benzothiazolinone hydrazone hydrochloride hydrate | Sigma-Aldrich | 129739-25G |
| DL-dithiothreitol | Sigma-Aldrich | D9163-1G |
| Corning microplate 96 well flat bottom | Fisher Scientific Ltd | TKT-521-050H |
| Ammonium iron (III) sulfate dodecahydrate | Sigma-Aldrich | F1668-250G |
| Sulfamic acid | Sigma-Aldrich | 242772-500G |
| Hydrochloric acid | Fisher Scientific Ltd | 12462-0026 |
Referanslar
- Carpita, N. C. Structure and Biogenesis of the Cell Walls of Grasses. Annu Rev Plant Physiol Plant Mol Biol. 47, 445-476 (1996).
- Gomez, L. D., Steele-King, C. G., McQueen-Mason, S. J. Sustainable liquid biofuels from biomass: the writing’s on the walls. New Phytol. 178, 473-485 (2008).
- Anthon, G. E., Barrett, D. M. Determination of reducing sugars with 3-methyl-2-benzothiazolinonehydrazone. Anal Biochem. 305, 287-289 (2002).
- Gomez, L. D., Whitehead, C., Barakate, A., Halpin, C., McQueen-Mason, S. J. Automated saccharification assay for determination of digestibility in plant materials. Biotechnol Biofuels. 3, 23-23 (2010).

