Recording of Local Field Potential from Both Hemispheres of the Mouse Brain
Abstract
Source: Chen, Y., et al. Evaluation of Hemisphere Lateralization with Bilateral Local Field Potential Recording in Secondary Motor Cortex of Mice. J. Vis. Exp. (2019).
This video demonstrates in vivo recording of local field potential (LFP) from bilateral M2 areas of the mouse brain. An anesthetized mouse is secured and prepared for the procedure. To record LFPs, insert microelectrodes into the target coordinates of the bilateral M2 areas, which are responsible for complex motor movements.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Animal anesthesia and surgery
- Weigh and anesthetize the mouse with your approved anesthesia regimen from your local animal care committee.
- Perform a tail or toe pinch with forceps to confirm deep anesthesia before surgery.
- Position the mouse in a stereotaxic apparatus and fix its head.
- Apply eye ointment on both eyes to keep them moist. Follow your local animal care guidelines regarding pre- and postoperative analgesia.
- Shave the hair using surgical clippers. Make a small incision (12-15 mm) in the middle of the exposed surgical area with scissors. Using forceps, gently pull the scalp away from the midline.
- Separate the skin gently and remove residual tissue. Clean the skull using hydrogen peroxide-coated cotton buds.
- Drill two small holes of radii 1.0-1.5 mm on both the skull's left and right sides to allow the recording microelectrodes to be inserted into the M2 regions under a stereomicroscope (Figure 1A).
NOTE: Stereotaxic locations of bilateral M2: 1.94 mm anterior to the bregma, 1.0 mm lateral to the midline, and 0.8-1.1 mm ventral to the dura. - Remove the dura mater carefully with a tungsten needle.
- Pull glass borosilicate micropipettes (outer diameter: 1.0 mm) as recording microelectrodes with resistance of 1-2 MΩ.
- Insert two separate recording microelectrodes filled with 0.5 M NaCl into the holes using mechanical micromanipulators (at 60°, Figure 1B).
2. LFP recordings in bilateral M2 of mice
- Lower the left and right glass electrodes slowly into the appropriate coordinates of bilateral M2 (Figure 1C).
- For quality control, test the resistance of each electrode using the differential amplifier before capturing LFPs.
- Set the recording process at 0.1 Hz high-pass and 1,000 Hz low-pass with 1,000x amplification.
- Collect digitized raw LFP data of at least 60 s spontaneous activities in a stable state, with mice breathing evenly at a respiratory rate of 2 breaths per second under anesthesia.
- After recording, slowly raise the electrodes out of the brain, then euthanize the mice by fast cervical dislocation.
- Save the data and analyze it offline.
Representative Results
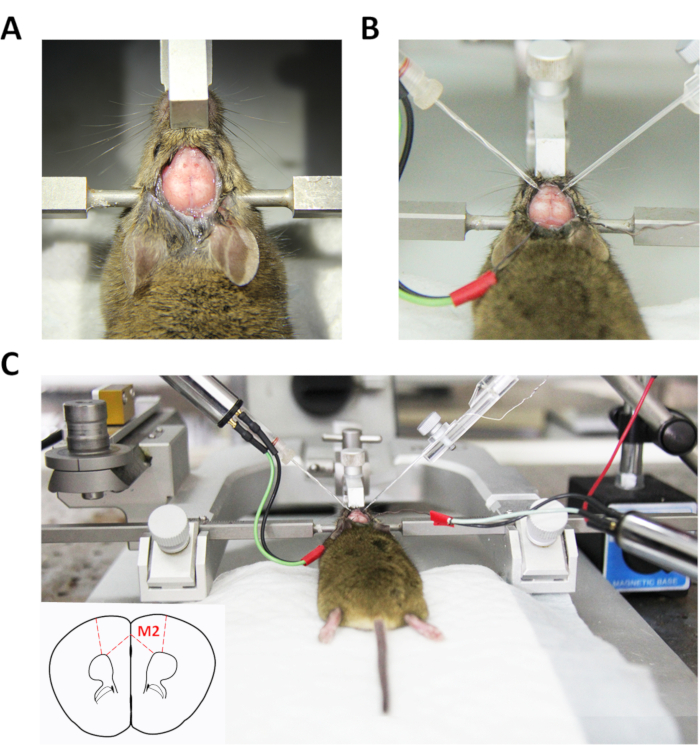
Figure 1: Diagram of the simultaneous LFP recording procedure. (A) Stereotaxic mouse with skull exposed and dura mater removed for in vivo bilateral recording of LFPs in left and right M2. (B) Two glass microelectrodes in touch with the cortical surface in the hole drilled simultaneously. (C) Recording microelectrodes and Ag/AgCl wires as reference electrodes positioned at appropriate sites.
Açıklamalar
The authors have nothing to disclose.
Materials
| AC/DC Differential Amplifier | A-M Systems | Model 3000 | |
| Analog Digital converter | Cambridge Electronic Design Ltd. | Micro1401 | |
| Glass borosilicate micropipettes | Nanjing spring teaching experimental equipment company | 161230 | Outer diameter: 1.0mm |
| Microelectrode puller | Narishige | PC-10 | |
| NaCl | Guangzhou Chemical Reagent Factory | 7647-14-5 | |
| Pin microelectrode holder | World Precision Instruments, INC. | MEH3SW10 | |
| Spike2 | Cambridge Electronic Design Ltd. | ||
| Stereomicroscope | Zeiss | 435064-9020-000 | |
| Stereotaxic apparatus | RWD Life Science | 68045 | |
| Urethane | Sigma-Aldrich | 94300 |

