Determining a Test Growth Hormone Effect on Kisspeptin Neurons using a Whole-Cell Patch Clamp
Abstract
Source: Silva, J. d. N., et al., Hypothalamic Kisspeptin Neurons as a Target for Whole-Cell Patch-Clamp Recordings. J. Vis. Exp. (2023)
This video demonstrates the use of the whole-cell patch-clamp technique to measure responses in kisspeptin neurons from transfected mouse hypothalamic slices. The process involves achieving whole-cell configuration and recording membrane potential changes induced by the test hormone using current-clamp mode.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Cell sealing for recording
- Ensure that all the pieces of equipment (microscope, amplifier, digitizer, micromanipulator, and others) are turned on before starting the recording.
- Fill the recording chamber, from a commercial brand (Table of Materials), attach to the microscope with the aCSF solution for recordings. Use a perfusion pump to constantly perfuse the aCSF at a rate of 2 mL/min.
- Transfer a brain slice of interest (one at a time) to the recording chamber. Use an acrylic transfer pipette (inverted Pasteur glass pipette attached to a silicone teat) to transfer the hypothalamic slices to the chamber. Use a slice anchor (Table of Materials) to hold the slice so it does not move during the aCSF perfusion.
- Place a slice at the center of the recording chamber attached to the microscope. The slice position is critical to allow a good view of the desired region under the microscope and for a perfect reach of the recording micropipette.
- Use an immersion microscope's low-power objective lens (10x or 20x) to assist in positioning the slice and locating the region of interest.
- After locating the region of interest, switch the objective lens to the high-power lens (63x) and focus on the tissue level, observing the endogenous fluorescent protein and shapes of the cells in the target region to locate the kisspeptin cells on the surface of the brain slice.
- When a possible target cell is located, mark it on the computer screen with the mouse cursor or by drawing a format, like a square, over the area of interest. The computer screen mark helps guide the recording micropipette's position to the cell.
- After determining the exact location of the target cell, lift the objective and introduce the recording micropipette filled with the internal solution. When placing the micropipette in the electrode holder, ensure that the internal solution is in contact with the silver electrode.
NOTE: For micropipette preparation, placement, and positioning on the electrode holder. - Apply positive pressure before submerging the micropipette in the aCSF solution, to prevent debris from entering the micropipette, using a 1-3 mL air-filled syringe connected to the micropipette holder through a polyethylene tubing (≈130 cm longer); apply nearly 100-200 μL of air.
- Using the micromanipulator, guide the micropipette below the center of the objective. Move the buttons on the micromanipulator to guide the micropipette on the X-Y-Z axis toward the cell of interest.
- Adjust the focus to see the tip of the micropipette and bring the focus closer, but not too close, to the slice. Reduce the speed of the micromanipulator and slowly lower the micropipette to the plane of focus. Ensure that the micropipette tip does not abruptly penetrate the slice, but rather slowly descends until it touches the surface of the cell/target region.
- Apply light positive pressure (≈100 μL) with the 1-3 mL air-filled syringe attached to the micropipette holder to clear any debris from the approach path.
- Focus on the target cell and slowly move the micromanipulator on the X-Y-Z axis to bring the micropipette closer to it. When touching the micropipette to the cell, a dimple caused by the pressure applied through the micropipette tip will be observed (Figure 2C).
- After forming the dimple, due to the micropipette's proximity to the cell, apply weak, brief suction by mouth (1-2 s) through the tube connected to the micropipette holder to generate the seal between the micropipette to the cell (gigaohm seal or gigaseal >1 GΩ; Figure 2D). To form the seal, use the voltage-clamp mode on the software.
- If the seal remains stable (the gigaohm seal should be mechanically stable and without noise interference, determined by observation for about 1 min), set the holding voltage at the closest physiological resting potential of the cell of interest. For kisspeptin hypothalamic neurons, -50 mV is recommended.
- Apply brief suction by mouth (negative pressure) with the micropipette sealed to the cell to break the plasma membrane (Figure 2E). Adequate whole-cell configuration is achieved when suction is performed with sufficient force so that the ruptured membrane does not clog the micropipette and does not attract a sizable portion of the membrane or even the cell.
- Check the system settings manual used. Use the software (see Table of Materials) to digitally check and calculate the series resistance (SR) and the whole-cell capacitance (WCC).
- On voltage-clamp mode, after breaking the cell membrane, enable the whole cell option, and click on the Auto command referring to the whole cell tab. The cell's SR and wcc will be automatically calculated and instantly displayed by the software. These parameters can also be checked by performing the membrane test with the amplifier mentioned in the Table of Materials.
- Make sure to check cell viability parameters. For kisspeptin neurons, check that the electrophysiological measurements are: SR < 25 mΩ, input resistance > 0.3 GΩ, and holding current absolute value < 30 pA. The mean value of the WCC of AVPV/PeNKisspeptin or ARHkisspeptin neurons is ≈ 10-12 pF in gonad-intact mice.
- Monitor the SR and the cell steady-state capacitance during the experiments. Ensure the SR does not change more than 20% during a recording and that the membrane capacitance is stable.
- Check the software settings. Create specific protocols for the recordings according to the type of experiment. To record membrane potential in the current-clamp mode, with equipment mentioned in the Table of Materials, low-pass filter the electrophysiological signals at 2-4 kHz and analyze results offline in software (see the Table of Materials for software information).
- Once the whole-cell configuration is properly achieved, measure synaptic currents in voltage-clamp mode (Figure 2G). Record changes in resting membrane potential (RMP) and induced RMP variations in the current-clamp mode (Figure 2H). Changes in RMP, such as depolarization of the cell membrane, can be induced by administering a known drug/neurotransmitter to the bath (described in step 1.2), as illustrated in Figure 1.
NOTE: In current-clamp mode, positive or negative current can be injected to hold the membrane voltage at a desired voltage. For kisspeptin neurons, we usually set zero current injection (I = 0) to record the spontaneous variation of the membrane potential.
Representative Results
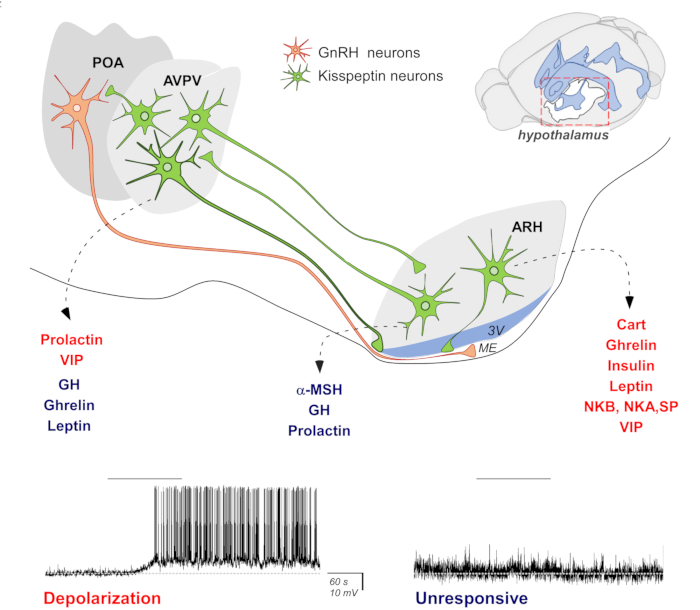
Figure 1: Schematic diagram summarizing the whole-cell patch-clamp technique's contribution to the knowledge of the kisspeptin neurons' activity. Kisspeptin neurons (shown in green) are located in the anteroventral periventricular and rostral periventricular nuclei (AVPV/PeN) and arcuate nucleus of the hypothalamus (ARH). The AVPV/PeNKisspeptin and ARHkisspeptin cells send direct connections to gonadotrophin-releasing hormone (GnRH) neurons' soma located in the preoptic area (POA) and their terminals at the median eminence (ME), culminating in the modulation of the hypothalamus-pituitary-axis (HPG). Different neuromodulators, such as hormones, have been shown to differentially modulate the activity of the AVPV/PeNKisspeptin and ARHkisspeptin neurons. Possible effects on the resting membrane potential are schematically demonstrated by representative tracings obtained using the whole-cell path-clamp technique and current-clamp recordings. The red color indicates that a specific neurotransmitter induces the depolarization of the resting membrane potential (RMP); the blue color indicates no effect on RMP. The dashed line indicates the RMP.
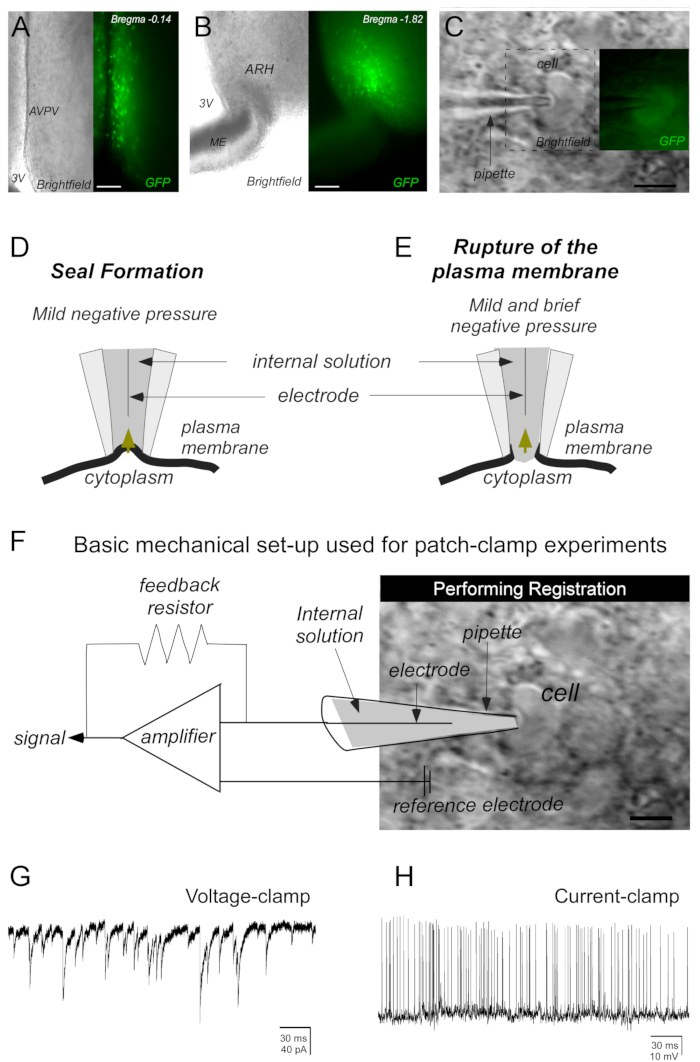
Figure 2: Basic steps to obtain the sealing of the cell of interest by the whole-cell patch-clamp technique. (A,B) Representative photomicrographs of brain slices (250 µm) containing kisspeptin cells at the anteroventral periventricular nucleus (AVPV) and arcuate nucleus of the hypothalamus (ARH). Kisspeptin neurons were identified by green fluorescent protein (GFP) expression. (C) Photomicrograph demonstrating a micropipette (containing electrolyte solution [internal solution]) close enough to the cell to create a dimple in the plasma membrane to perform the seal. (D,E) Mild negative pressure (mouth suction performed on a tube attached to the headstage and micropipette) is required to seal the cell membrane to the micropipette (D). A second application of negative pressure (mild and brief) is necessary to induce the plasma membrane rupture (E). (F) The registration of the cell activity is performed by a mechanical setup used for patch-clamp experiments. After breaking the plasma membrane, currents flowing through the ionic channels in the patched cell can be recorded by an electrode connected to a highly sensitive amplifier. A feedback resistor generates the current needed for voltage-clamp (G) or current-clamp (H) recordings. Abbreviations: 3V = third ventricle; ME = median eminence. Scale bars: A = 130 µm, B = 145 µm, C = 20 µm, D = 15 µm.
Açıklamalar
The authors have nothing to disclose.
Materials
| Compounds for aCSF, internal and slicing solutions | |||
| ATP | Sigma Aldrich/various | A9187 | |
| Calcium chloride | Sigma Aldrich/various | C7902 | |
| D-(+)-Glucose | Sigma Aldrich/various | G7021 | |
| EGTA | Sigma Aldrich/various | O3777 | |
| HEPES | Sigma Aldrich/various | H3375 | |
| KCl | Sigma Aldrich/various | P5405 | |
| K-gluconate | Sigma Aldrich/various | G4500 | |
| KOH | Sigma Aldrich/various | P5958 | |
| Magnesium chloride | Sigma Aldrich/various | M9272 | |
| Magnesium Sulfate | Sigma Aldrich/various | 230391 | |
| NaCl | Sigma Aldrich/various | S5886 | |
| Monosodium phosphate | Sigma Aldrich/various | S5011 | |
| Sodium bicarbonate | Sigma Aldrich/various | S5761 | |
| Equipments | |||
| Amplifier | Molecular Devices | Multiclamp 700B | |
| DIGIDATA 1440 LOW-NOISE DATA ACQUISITION SYSTEM | Molecular Devices | DD1440 | |
| Digital peristaltic pump | Ismatec | ISM833C | |
| Faraday cage | TMC | 81-333-03 | |
| Imaging Camera | Leica | DFC 365 FX | |
| Micromanipulator | Sutter Instruments | Roe-200 | |
| Micropipette Puller | Narishige | PC-10 | |
| Microscope | Leica | DM6000 FS | |
| Recovery chamber | Warner Instruments/Harvard apparatus | – | |
| Recording chamber | Warner Instruments | 640277 | |
| Spatula | Fisher Scientific /various | FISH-14-375-10; FISH-21-401-20 | |
| Software and systems | |||
| AxoScope 10 software | Molecular Devices | – | Commander Software |
| LAS X wide field system | Leica | – | Image acquisition and analysis |
| MultiClamp 700B | Molecular Devices | MULTICLAMP 700B | Commander Software |
| PCLAMP 10 SOFTWARE FOR WINDOWS | Molecular Devices | Pclamp 10 Standard | |
| Tools | |||
| Ag/AgCl electrode, pellet, 1.0 mm | Warner Instruments | 64-1309 | |
| Slice Anchor | Warner Instruments | 64-0246 |

