Photostimulation and Whole-Cell Patch Clamp Recordings of Neurons in Mouse Hippocampal Slices
Abstract
Source: Richevaux, L., et al. In Vivo Intracerebral Stereotaxic Injections for Optogenetic Stimulation of Long-Range Inputs in Mouse Brain Slices. J. Vis. Exp. (2019)
The video demonstrates a protocol to study synaptic interactions and neuronal responses in a transfected mouse hippocampal brain slice. It involves illuminating long-range sensory neurons to trigger action potentials and recording the resulting excitatory post-synaptic potentials in the recorded neuron using the whole-cell patch-clamp technique.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Solutions for acute slice recordings
- Prepare stock solutions of 10x artificial cerebrospinal fluid (aCSF) solution (124 mM NaCl, 2.5 mM KCl, 26 mM NaHCO3, 1 mM NaH2PO4, and 11 mM D-glucose) in pure deionized water prior to electrophysiology experiments. Store the solution at 4 °C in 1 L bottle without CaCl2 and MgCl2.
- Prepare the potassium-gluconate-based pipette solution to contain 135 mM K-gluconate, 1.2 mM KCl, 10 mM HEPES, 0.2 mM EGTA, 2 mM MgCl2, 4 mM MgATP, 0.4 mM Tris-GTP, 10 mM Na2-phosphocreatine, and 2.7–7.1 mM biocytin for post-hoc cell morphology revelation. Adjust the solution's pH to 7.3 and osmolarity to 290 mOsm. Store 1 mL aliquots at -20 °C.
2. Whole-cell patch-clamp recording
- Gently transfer a brain slice containing the hippocampal complex with a custom-made glass transfer pipette to the recording chamber mounted on an upright microscope. A transfer pipette is made of a shortened Pasteur pipette (inner diameter 6.5 mm) attached to a rubber pipette bulb. Continuously perfuse the recording chamber (3 mL) with 34 °C (warmed) aCSF bubbled with 95%/5% O2/CO2. Set the speed of the peristaltic pump to 2-3 mL/min.
- Briefly examine Chronos-GFP expression in axon terminals in the region of interest with blue LED illumination (470 nm) and observe with a 4x objective. GFP fluorescence is visualized through an appropriate emission filter, with a CCD camera image displayed on a computer screen.
- Place a slice anchor made from a U-shaped platinum wire with tightly spaced nylon strings ("harp") on the slice to maintain it.
- Change to a 63x immersion objective and adjust the focus. Check for axons expressing Chronos-GFP and choose a pyramidal neuron for patch recording.
- Move the objective upward.
- Pull pipettes using a Brown-Flaming electrode puller from borosilicate glass. The puller is set to produce pipettes with approximately 1 μm in tip diameter. Fill the pipettes with K-gluconate-based internal solution.
- Mount the pipette in the pipette holder on the head stage. Lower the pipette in the chamber and find the tip under the objective. Pipette resistance should be between 3–8 MΩ. Apply a light positive pressure with a syringe so as to see a cone of solution outflow out of the pipette and progressively lower the pipette and objective to the surface of the slice.
- Patch the cell in voltage-clamp configuration: approach the identified neuron and delicately press the pipette tip onto the soma. The positive pressure should produce a dimple on the membrane surface. Release the pressure to create a giga-ohm seal (>1 GΩ resistance). Once sealed, set the holding voltage to -65 mV. Break the membrane with a sharp pulse of negative pressure: this is achieved by applying strong suction to a tube connected to the pipette holder.
- Record in whole-cell current clamp mode the responses of the neuron to hyperpolarizing and depolarizing current steps (Figure 1A).
NOTE: This protocol will be used to determine the active and passive intrinsic properties of the cell. Custom-written MATLAB routines are used for off-line analysis. - Record in current- or voltage-clamp postsynaptic responses to whole-field 475 nm LED stimulation of afferent fibers expressing Chronos. Stimulate with trains of 10 stimulations of 2 ms durations at 20 Hz (Figure 1 B, C). Light intensity may vary from 0.1–2 mW.
NOTE: Light intensity was measured with a digital handheld optical power console equipped with a photodiode sensor, positioned under the objective. Response latencies of 2–4 ms are characteristic for a monosynaptic connection.
Representative Results
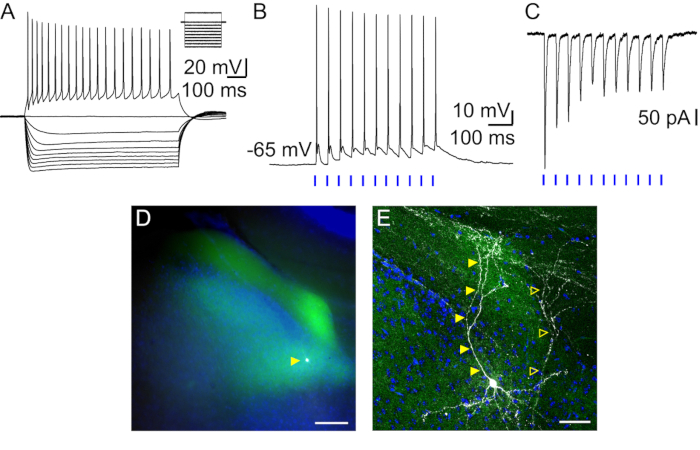
Figure 1: Presubicular layer III neuron: intrinsic properties, response to light stimulation of thalamic afferents, and post-hoc revelation of cell morphology. (A) Firing pattern and membrane potential variations of layer III neuron for hyperpolarizing and depolarizing current steps. (B, C) Responses of layer III neuron to 2 ms light stimulations (blue bars) of thalamic axons recorded in (B) current-clamp and (C) voltage-clamp modes. (D, E) Layer III pyramidal neuron (white, indicated by filled yellow triangle) surrounded by thalamic axons expressing Chronos-GFP (green) in presubicular superficial layers with DAPI staining (blue) in horizontal slice imaged with an epifluorescence microscope (D, scale bar = 100 µm) and confocal microscope at a high magnification (E, scale bar = 50 µm). The cell in (A) is indicated with filled yellow triangles. A second, partially filled neuron is present in this slice indicated with empty yellow triangles.
Açıklamalar
The authors have nothing to disclose.
Materials
| KCl | Sigma | P4504 | final 1.2 mM |
| Magnesium chloride | Sigma | 63069 | final 2 mM |
| MultiClamp 700B | Axon Instruments | ||
| pClamp acquisition software | Axon Instruments | ||
| Peristaltic pump | Gilson | Minipuls 3 | 14-16 on the display for 2-3 ml/min |
| Potassium gluconate (K-gluconate) | Sigma | G4500 | Final 135 mM |
| Borosilicate Capillaries | Havard Apparatus | GC150-10 | 1.5 mm outer, 0.86 inner diameter |
| Brown Flaming electrode puller | Sutter Instruments | P-87 | |
| EGTA | Sigma | E4368 | Final 0.2 mM |
| 470 nm LED | Cairn Research | P1105/470/LED DC/59022m | Use with matched excitation filter 470/40x and emission filter for GFP |
| AAV5.Syn.Chronos-GFP.WPRE.bGH | Penn Vector Core | AV-5-PV3446 | Lot V6026R, qTiter GC/ml 4.912e12, ddTiter GC/ml 2.456e13 |
| CCD Camera | Andor | DL-604M | |
| Confocal Microscope | Zeiss | LSM710 | 20X |
| Digidata 1440A | Axon Instruments | ||
| Digital handheld optical meter | ThorLabs | PM100D | Parametered on 475 nm |
| HEPES | Sigma | H3375 | Final 10 mM |
| LED Power Supply | Cairn Research | OptoLED Light Source |

