Recording Whole-Cell Currents with GABA Puffing in a Mouse Brain Slice
Abstract
Source: Feng, Y. et al., Whole-cell Currents Induced by Puff Application of GABA in Brain Slices. J. Vis. Exp. (2017)
This video demonstrates the recording of whole-cell currents induced by gamma-aminobutyric acid, or GABA, in a mouse prefrontal cortical brain slice. Controlled delivery of GABA opens chloride channels in a postsynaptic neuron, generating inhibitory postsynaptic currents, or IPSCs. These IPSCs increase with higher GABA concentrations, reflecting GABA's inhibitory activity on synaptic transmission.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Preparation of Solutions
- Prepare 500 mL of standard artificial cerebrospinal fluid (ACSF) containing the components described in our published work as listed in Table 1.
- Use clean glass beakers with deionized water to prepare a fresh ACSF solution. Clean spatulas with deionized water and dry before using to add sodium chloride (NaCl), potassium chloride (KCl), Di-hydrogen sodium phosphate (NaH2PO4), sodium bi-carbonate (NaHCO3), magnesium sulfate (MgSO4), D-glucose, and add the calcium chloride (CaCl2) after having bubbled the solution with 95% O2 / 5% CO2 mixture for ~20 min (as detailed in Table 1) to 500 mL of deionized water. Stir until fully dissolved.
- Ensure the ACSF is completely clear without any precipitation. Then, adjust the pH to 7.2-7.4. Bubble with a gas mixture composed of 95% O2 and 5% CO2 for ~20 min.
- Prepare 250 mL of slice-cutting solution containing the chemicals described in Table 2.
- Add KCl, magnesium chloride (MgCl2), NaH2PO4, NaHCO3, choline·Cl, and D-glucose and add the calcium chloride (CaCl2) last as indicated in 1.1.1, to 250 mL of deionized water and dissolve as described in 1.1.1 and 1.1.2.
- After bubbling with 95% O2/5% CO2, place the solution in a freezer for 15-20 min so that the cutting solution becomes ice-cold and partially frozen.
NOTE: We use this cutting solution when making slices from 1-2 month-old mice; the formula may differ for mice older than 2 months. - Prepare a lipopolysaccharide (LPS) solution by dissolving it in sterile endotoxin-free isotonic saline (0.9% NaCl). LPS is administered intraperitoneally (0.5 mg/kg) and begins recording 2 h later.
2. Preparation of Prefrontal Cortex Slice
- Take the cutting solution out of the freezer and homogenize to obtain a slush. Place the solution on ice and bubble constantly with 95% O2/5% CO2.
- Using large, sharp scissors, quickly decapitate a mouse as described and immediately put the head into a beaker filled with ice-cold (0-4°C) cutting solution.
- Cut the top of the skull along the midline in a caudal-to-nasal direction with fine scissors and remove the lateral skull portions. Remove the brain with a fine spatula and drop into ice-cold cutting solution.
NOTE: Steps 2.2-2.3 must be done rapidly (ideally < 1 min). - Place the brain on the lid of a 9 cm Petri dish filled with ice. Rinse the brain with ice-cold cutting solution. Cut off the cerebellum and olfactory bulb with a razor blade.
- Apply cyanoacrylate glue to the specimen holder of a vibratome. Carefully place the brain block on the drop of glue, rostral side up, and immediately immerse in ice-cold cutting solution.
- Adjust the blade holder of the vibratome to an angle of 15° with reference to the horizontal plane and prepare 350-µm coronal brain slices (blade vibration frequency = 85 Hz, speed = 0.1 mm/s).
- Using a pipette, transfer the slices into the recovery chamber filled with ACSF, incubate for 1 h (at RT) with constant bubbling of 95% O2/5% CO2.
3. Whole-cell Patch Recording
- Make glass borosilicate micropipettes for use as recording electrodes or puff micropipettes according to the specific guidelines in the Puller Operation Manual. For recording electrodes, tip resistances are in the range 4-6 MΩ, while puff micropipettes have tip diameters in the range 2-5 µm.
- Add Na+ channel blocker (Tetrodotoxin (TTX), 1 µM), to block fast Na+ channel and thus action potentials, to ACSF and maintain a constant flow rate of this solution of 2-3 mL/min in the recording chamber, by peristaltic pump into the chamber and aspiration out of it. Bubble the ACSF with 95% O2/5% CO2 constantly to ensure the viability of the slices.
- Transfer a brain slice into the recording chamber using a modified Pasteur pipette whose fine tip was cut to fit the slice size. Cover the slice with a platinum slice anchor with parallel nylon threads spaced ≥2 mm apart to hold the slice on the platform.
- Fill the recording micropipette with internal solution (Table 3) and fill the puff micropipettes with gamma-aminobutyric acid (GABA) at 10 µM, 50 µM, and 100 µM (dissolved in ACSF), or ACSF as vehicle control.
- To prevent blockage with debris, apply light positive pressure using a 1 mL syringe before immersing the recording electrode in the ACSF. Using a micromanipulator under a microscope (4x objective lens), position the recording electrode and puff micropipette so that the tips appear in the center of the video image in the monitor (Figure 1A).
- Adjust the microscope focus (40x objective lens) while gradually lowering the puff micropipette and place it above the recording neuron at an angle of 45°. Keep the distance between the tip of the puff micropipette and the neuron recorded between 20-40 µm (Figure 1B). Slowly and carefully approach the neuron with the recording electrode and then release the positive pressure. Apply a weak and brief suction through the tubing connected to the electrode holder to create a giga-ohm seal.
- Maintain the voltage at 0 mV. After formation of the giga-ohm seal, compensate for fast and slow capacitance manually or automatically. Then apply a brief and strong suction through the tubing mentioned above to get into whole-cell mode.
- Record eIPSC in voltage-clamp mode. Apply low gas pressure (nitrogen, 4 – 6 psi) to the puff micropipette using a Picospritzer controlled by a Master 8 voltage step generator to deliver single or paired GABA puffs (Figure 2).
Table 1. Recording ACSF.
| Substance | g/mol | Concentration (mM) | Weight (g/L) |
| NaCl | 58.443 | 119 | 6.9547 |
| KCl | 74.551 | 2.5 | 0.1864 |
| NaH2PO4 | 137.99 | 1 | 0.1380 |
| NaHCO3 | 84.007 | 26.2 | 2.2010 |
| MgSO4 | 120.366 | 1.3 | 0.1565 |
| D-glucose | 198.17 | 11 | 2.1799 |
| CaCl2 | 110.98 | 2.5 | 0.2775 |
Table 2. Slice cutting solution.
| Substance | g/mol | Concentration (mM) | Weight (g/L) |
| KCl | 74.551 | 2.5 | 0.1864 |
| MgCl2 | 95.211 | 7 | 0.6665 |
| NaH2PO4 | 137.99 | 1.25 | 0.1725 |
| NaHCO3 | 84.007 | 25 | 2.1002 |
| Choline·Cl | 139.63 | 115 | 16.0575 |
| D-glucose | 198.17 | 7 | 1.3872 |
| CaCl2 | 110.98 | 0.5 | 0.0555 |
Table 3. Internal solution for whole-cell patch recordings.
| Substance | g/mol | Concentration (mM) | Weight (g/L) |
| Cs gluconate | 328.052 | 100 | 32.8052 |
| CsCl | 168.36 | 5 | 0.8418 |
| HEPES | 238.301 | 10 | 2.3830 |
| MgCl2 | 95.211 | 2 | 0.1904 |
| CaCl2 | 110.98 | 1 | 0.1110 |
| BAPTA | 476.433 | 11 | 5.2408 |
| Na2ATP | 551.1 | 4 | 2.2044 |
| Na2GTP | 523.2 | 0.4 | 0.2093 |
Representative Results
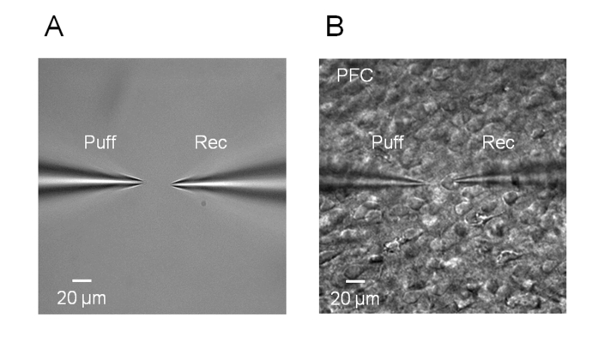
Figure 1. Diagram of the puff micropipette and recording electrode.
(A) The distance between the puff and recording micropipettes. (B) The puff micropipette (left) and the recording electrode (right) on the surface of a brain slice.
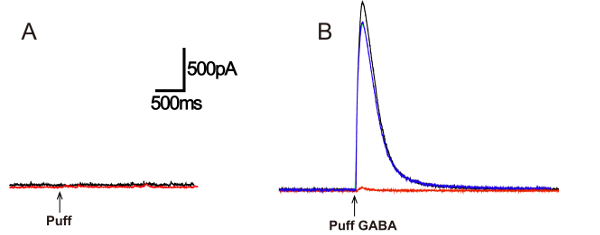
Figure 2. Picrotoxin blockade of IPSC induced by GABA puff.
(A) Puffing ACSF and sucrose (50 µM) induces no response. (B) Puffing 50 µM GABA induces an IPSC (black) (puff duration = 200 ms, 4-6 psi) in ACSF containing TTX (1 µM), CNQX (20 µM) and AP5 (50 µM) to block action potentials and excitatory current mediated by AMPA and NMDA receptors. The GABA-induced IPSC is blocked by bath application of a GABA receptor inhibitor, 100 µM picrotoxin (red). The IPSC recovers after wash-out of picrotoxin.
Açıklamalar
The authors have nothing to disclose.
Materials
| Glass Borosilicate micropipettes | Shutter Instruments | BF150-86-10 | 1.50 mm outer diameter; 0.86 mm inner diameter |
| Micropipette Puller | Shutter Instruments | MODEL P-97 | Flaming/Brow Micropipette Puller |
| Micromanipulators | Shutter Instruments | MP-285 | |
| Computer controlled Amplifier | Molecular Devices | Multiclamp 700B | |
| Digital Acquisition system | Molecular Devices | Digidata 1440A | |
| Imaging Camera | Nikon | 2115001 | Inspection equipment |
| Microscopy | Nikon | Eclipse FN1 | |
| Master 8 | A.M.P.I. | Master-8 Pulse stimulator | |
| Vibratome Slicer | Leica | VT 1000S | |
Picospritzer  |
Parker Hannifin | Pressure Systems for Ejection of Picoliter Volumes in Cell Research | |
| Razor blade | Gillette | 74-S | FLYING EAGLE |
| Video monitor | Panasonic | WV-BM 1410 | |
| 502 Glue | Deli | 7146 | Cyanoacrylate Glue |
| Peristaltic pump | Shanghai JIA PENG Corporation | BT100-1F | |
| Video Camera | Olympus America Medical | OLY-150 | |
| Transfer Pipets | Biologix | 30-0138A1 |

