Frozen Skin Tissue Block Preparation: A Protocol to Preserve Cutaneous Melanoma Samples from Murine Dorsal Skin
Abstract
Source: Moon, H., et al. Spatial and Temporal Control of Murine Melanoma Initiation from Mutant Melanocyte Stem Cells. J. Vis. Exp. (2019)
This video describes the detailed protocol for isolating melanoma-containing murine dorsal skin, followed by cryo-embedding to prepare a frozen skin tissue block. The cryo-embedded tissue block can be sectioned and microscopically examined to study melanoma progression.
Protocol
All procedures involving animals have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Tissue Processing
- Skin isolation
- Obtain the following prior to starting: necropsy instruments, filter paper, and paper towels.
- Euthanize the mice according to approved protocols, and confirm death prior to proceeding.
- Using a hair clipper, shave any hair from the dorsal skin area. Lightly brush the area with a lint-free tissue to clear the area.
- Make a small incision with sharp scissors just above the base of the tail.
- Insert large blunt scissors into the incision and separate the subdermal connective tissues.
- Carefully isolate the skin region of interest by cutting with sharp scissors along the edge of the tissue.
- Place the skin tissue onto a clean paper towel and use dull forceps to stretch the skin so that it adheres to the towel and is taut. This step serves to provide additional support for the tissue so that it can be manipulated and processed while maintaining its shape.
NOTE: Be careful to only handle the outside regions of the skin tissue, as the forceps may cause damage to the skin which can be seen histologically.
- Tissue fixation
- Fold a piece of filter paper in half and label appropriately. Place the skin along with paper towel backing into the folded paper immediately adjacent to the crease, ensuring that the sample is smooth and not folded or crumpled. Seal the filter paper by stapling the open sides, and then trim so that the remaining paper can be used for future samples.
NOTE: This step is performed so that the skin retains its shape during fixation. - Fully submerge the tissue sample in 10% neutral buffered formalin for 3 to 5 h at room temperature or overnight at 4 °C.
- Following fixation, remove the samples from formalin, wash in deionized water (2x 5 min each) and proceed to make tissue blocks. Carefully remove any residual material from the skin tissue (i.e., residual paper).
- Fold a piece of filter paper in half and label appropriately. Place the skin along with paper towel backing into the folded paper immediately adjacent to the crease, ensuring that the sample is smooth and not folded or crumpled. Seal the filter paper by stapling the open sides, and then trim so that the remaining paper can be used for future samples.
- Making frozen tissue blocks (Figure 1)
- Trim the edges of the skin sample so that they are not jagged and then make 3 sagittal cuts. The width of these strips should not be more than the height of the cryomold (5 mm). Next, make transverse cuts to have pieces of skin that are not longer than the width of the cryomold (20 mm) and can be embedded. Repeat with the remaining half of dorsal skin, or save the tissue for other uses (alternately, save half prior to fixation).
NOTE: It's important to keep skin pieces in the proper orientation. - Orient the cryomold (25 x 20 x 5 mm3) in portrait orientation and place 4 to 5 pieces of skin on top of the OCT. Once all pieces are in place, use 2 pairs of fine forceps to bring the long edge of the skin to the bottom of the cryomold. The skin should now be standing up in the OCT perpendicular to the base of the mold. Take care to minimize the formation of air pockets within the OCT during this step (Figure 1).
- Fill the mold with OCT to the second lip, and place it on the flat surface of dry ice. Use forceps to adjust any skin pieces that may have shifted during transfer as the block begins to freeze. Once the bottom layer is solidified, manipulation of tissues will be impossible.
- Cut 8-10 µm slides for analysis.
- Trim the edges of the skin sample so that they are not jagged and then make 3 sagittal cuts. The width of these strips should not be more than the height of the cryomold (5 mm). Next, make transverse cuts to have pieces of skin that are not longer than the width of the cryomold (20 mm) and can be embedded. Repeat with the remaining half of dorsal skin, or save the tissue for other uses (alternately, save half prior to fixation).
- Making Formalin Fixed Paraffin Embedded (FFPE) tissue blocks
- Place 2 pieces of fixed skin into a labeled cassette and dehydrate in increasing concentrations of ethanol (75% ethanol for 30 min, 85% for 30 min, 90% for 30 min, 95% for 30 min, 100% for 2x 30 min, Xylene or xylene substitute for 2x 30 min). Incubate with Paraffin at 58-60˚ C, first for 30 min and then overnight.
- Embed the tissue in a 24 x 24 mm2 stainless steel tissue mold with liquid paraffin and solidify at -5 °C. The tissue orientation should be similar to that of frozen tissue blocks so that longitudinal sections across multiple hair follicles can be visualized (Figure 1).
- Once the paraffin has solidified, remove the mold and cut it into 5-10 μm sections for analysis.
Representative Results
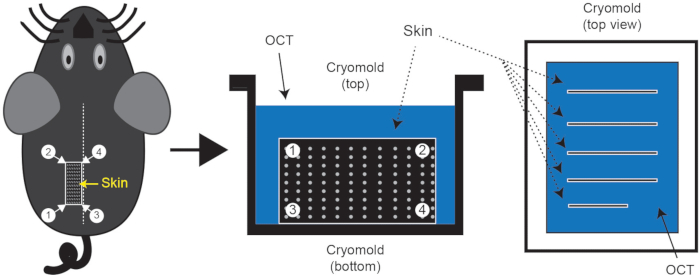
Figure 1: Skin tissue isolation and cryo-embedding. Following euthanasia, shave the mouse dorsal skin region of interest and embed skin in cryomold containing OCT. The dotted line on the mouse represents the midline. Typically, 3 or 4 pieces of skin can be isolated with line segment 3-4 along the midline; one such piece is indicated by a rectangle 1/2/3/4. These skin pieces should be embedded in the OCT as shown in the figure.
Açıklamalar
The authors have nothing to disclose.
Materials
| Pet hair trimmer | Wahl | 09990-502 | |
| Hair removal cream | Nair | n/a | Available at most drug stores |
| Cotton swabs | various | ||
| Ultraviolet light bulb | UVP | 95-0042-08 | model XX-15M midrange UV lamp |
| 200 proof ethanol | various | pure ethanol | |
| Histoplast PE | Fisher Scientific | 22900700 | paraffin pellets |
| Neutral Buffered Formalin, 10% | Sigma | HT501128-4L | |
| O.C.T. Compound | Thermo | 23730571 | |
| Tissue Cassette | Sakura | 89199-430 | for FFPE processing |
| Cryomolds | Sakura | 4557 | 25 x 20 mm |
| FFPE metal mold | Leica | 3803082 | 24 mm x 24 mm |
| Isoflurane | various | Veterinary grade | |
| Anesthesia inhalation system | various | Veterinary grade | |
| Fine scissor | FST | 14085-09 | Straight, sharp/sharp |
| Fine scissor | FST | 14558-09 | Straight, sharp/sharp |
| Forcep | FST | 11252-00 | Dumont #5 |
| Forcep | FST | 11018-12 | Micro-Adson |
| Tyr-CreER; LSL-BrafV600E; Pten f/f | Jackson Labs | 13590 | |
| LSL-tdTomato | Jackson Labs | 7914 | ai14 |

