Two-Photon Laser-Induced Neural Injury: A Method to Observe Axon Degeneration and Regeneration in Drosophila Larvae
Abstract
Source: Li, D., et al. A Drosophila In Vivo Injury Model for Studying Neuroregeneration in the Peripheral and Central Nervous System. J. Vis. Exp. (2018).
This video describes two-photon laser ablation of axons in a Drosophila melanogaster larva and an example protocol to induce injury and image the injury site.
Protocol
This protocol is an excerpt from Li et al., A Drosophila In Vivo Injury Model for Studying Neuroregeneration in the Peripheral and Central Nervous System, J. Vis. Exp. (2018).
1. Two-photon Injury and Confocal Imaging
- Microscope setup
NOTE: A confocal laser scanning microscope with a two-photon laser was used for this experiment, but other systems with an equivalent setup will also suffice. The two-photon laser (930 nm) was used for delivering injury and an Argon laser (488 nm) was used for confocal imaging of GFP.- At the beginning of each session, turn on the two-photon laser and/or the confocal laser(s), and the microscope. Open the imaging software.
- For two-photon injury, set up the following parameters for imaging GFP with the two-photon laser at 930 nm (1,950 mW).
- Select line scan mode. Open up the pinhole all the way. Increase laser intensity to ~20% (390 mW).
- Select 512 x 512 as the frame scan. Use maximal scanning speed (typically with the pixel dwell time at 0.77 µs). Ensure that the average number is 1 and bit depth is 8 bits.
- Set gain to ~750, and the offset to 0.
- Save this pre-set experimental protocol as 2P GFP 930 Ablation, allowing for easy reuse in future experiments.
- For confocal imaging, set up the following parameters for imaging GFP with the Argon laser at 488 nm:
- Select the Acquisition tab and then Z-stack.
- Under "Laser", turn on the power for the 488 nm Argon laser.
- Go to Channels, select the 488 mm laser, and increase the laser power to 5-10%. For the pinhole, use the 1-2 airy unit (AU). Adjust the gain to 650.
- In Acquisition Mode, select 1024 x 1024 as the frame scan, use the maximum scan speed, an average number of 2, and bit depth of 8 Bit.
- Save this pre-set experimental protocol as GFP Imaging.
- Larvae anesthesia with diethyl ether and mounting
- In a fume hood, place a 60-mm glass dish in a 15-cm plastic Petri dish. Fold and lay a piece of tissue paper at the bottom of the glass dish, then place a grape juice agar plate on the tissue. Add diethyl ether into the glass dish, to the point where the tissue paper is soaked and there is a layer of liquid ether remaining in the dish. Keep the lid on at all times.
- Prepare a glass slide with one drop of halocarbon 27 oil in the center. Add 4 spots of vacuum grease onto the four corners of the slide, to later support the coverslip.
- Use forceps to pick up a cleaned larva and place it on the agar plate in the 60-mm glass dish. Cover the glass dish with its lid and wait until the larva stops moving. For PNS injury/imaging, take out the larva as soon as its tail stops twitching. For the CNS, wait until the entire larva becomes motionless, especially the head segments.
NOTE: The timing of ether exposure is critical. See Discussion. - Carefully pick up the anesthetized larva and place it head-upright into the drop of halocarbon oil on the slide. Add a coverslip on top of the slide. Use gentle pressure to push down on the coverslip, until it touches the larva (Figure 1A).
- Adjust the larva's position by gently pushing the coverslip towards the left or right to roll the larva, so that the neuron/axon/dendrite of interest is on the top and closest to the microscope lens.
- For PNS injury, mount the larva dorsal side up, so that both the tracheas are visible. Then roll the larva ~30 degrees to the left for injuring class III da neuron axons (Figure 1B and 1C), 90 degrees for injuring class IV da neuron axons (Figure 1B and 1E), or ~30 degrees for injuring class IV da neuron dendrites (Figure 2A).
- For CNS injury, position the larva to be perfectly ventral side up (Figure 3A), so that the region of interest is closest to the microscope lens in the z-plane.
- Injury by two-photon laser
- Place the slide with the larva under the microscope and secure it in place with the slide holder on the stage. Use the 10X (0.3 NA) objective to find the larva.
- Add 1 drop of objective oil onto the coverslip, switch to the 40X (1.3 NA) objective and adjust the focus.
- Switch to the scanning mode and reuse the experimental protocol 2P GFP 930 Ablation. Make sure the pinhole is opened all the way.
NOTE: The configuration needs to be optimized based on individual systems. - Start the Live mode to locate the region of interest (ROI), and fine-tune the settings to achieve good image quality with the appropriate zoom.
NOTE: The purpose of this step is to find the neuron/axon/dendrite to injure, rather than taking the best quality image. Therefore, use the minimal settings sufficient to visualize the target area, in order to avoid overexposure or photobleaching. - Stop Live scan, so that the Crop button will become available. Let the still image serve as the roadmap. Select the Crop function and adjust the scan window to focus on the target to be injured.
- Reduce the ROI to be the size of the prospective site of injury. For example, just cover the width of an axon or a dendrite, to ensure the precision of the injury and reduce damage to neighboring tissues. If desired, zoom in on the ROI before cropping, allowing for more precise injury.
- Open a new imaging window. Reduce the scan speed and increase laser intensity. Determine the increase in laser intensity based on the tissue fluorescence signal scanned in Live mode.
- Typically, set the two-photon laser intensity starting from 25% for PNS injury and 50-100% for VNC injury. For PNS axon injury, ensure that the laser intensity is ~480 mW and pixel dwell time is 8.19 μs. For the VNC axon injury, ensure that the laser intensity and pixel dwell time are usually 965-1930 mW and 8.19-32.77 μs, respectively.
- Start Continuous scan. Leave the cursor hovering over the Continuous button. Keep a close eye on the image and stop the scan as soon as a drastic increase in fluorescence is observed.
NOTE: The appearance of the fluorescence spike is due to auto-fluorescence at the injury site. - Switch back to the Live mode by reusing the settings. Find the region of interest that was just targeted by adjusting the focus.
NOTE: A good indication of successful injury is the appearance of a small crater, ring-like structure, or localized debris right at the injury site. - Move to the next neuron and repeat from Step 1.3.5, to injure multiple neurons in a single animal. Or repeat Step 1.3.5 while gradually increasing the power and/or reducing scan speed if the initial injury was insufficient.
NOTE: In the case that the laser power is too high, a large damaged area will be visible in the post-injury live scan image. Too much injury may cause the death of the larva. - Recover the larva by carefully removing the coverslip and transferring the injured larva onto a new plate with yeast paste. Ditch several caves on the agar plate with forceps; alternatively, make an island of agar in the plate instead of using the whole plate, to reduce the possibility of the larva crawling out of the plate.
- Put the plate in a 60-mm Petri dish with wet tissue (soaked with 0.5% propionic acid solution) and culture at room temperature or 25 °C.
NOTE: The larva will remain in the larval stage for approximately an extra day at room temperature (22 °C) compared to at 25 °C.
- Post-injury confocal imaging
- Image the injured larva at desired time points by preparing the larva using the same procedure of anesthesia and mounting as in Step 1.2, then imaging with the confocal laser.
NOTE: Image the larva at 24 h after injury (AI) to confirm axonal injury and at 48 h AI (class IV da neurons) or 72 h AI (class III da neurons) to assess regeneration. - Locate the larva using the 10X objective, then switch to a 25X (0.8 NA) objective. Reuse the experimental protocol GFP Imaging.
- Click the Live button and find the same neuron injured previously.
- Set the first and last Z positions in the live scan window. Press stop and click Start experiment to acquire a Z-stack image.
NOTE: Make sure that a normalization point (the axon converging point) is included when capturing images so that quantification of regeneration is possible (Figure 1D, 1F) – this is discussed further in the data analysis section. - Switch to Image Processing, select the image just taken, and generate a maximum intensity projection. Save both the z-stack and maximum intensity projection images.
- Image the injured larva at desired time points by preparing the larva using the same procedure of anesthesia and mounting as in Step 1.2, then imaging with the confocal laser.
Representative Results
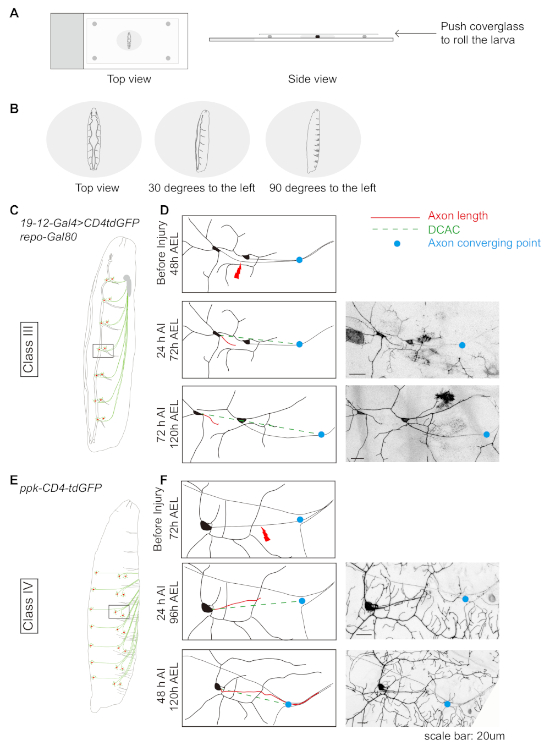
Figure 1: Da neuron axon regeneration in the periphery displays class specificity. (A and B) Schematic drawing showing the position of the larvae. (C) Schematic drawing of class III da neurons. (D) Axons of the class III da neurons ddaF, labeled with 19-12-Gal4, UAS-CD4-tdGFP, repo-Gal80/+, fail to regrow. (E) Schematic drawing of class IV da neurons. (F) Axons of the class IV da neurons v'ada, labeled with ppk-CD4-tdGFP/+, regrow beyond the lesion site. (D and F) Red line indicates axon length while green dashed line marks the distance between the cell body and the axon converging point (DCAC). The blue dot marks the axon converging point. Scale bar = 20 µm. Please click here to view a larger version of this figure.
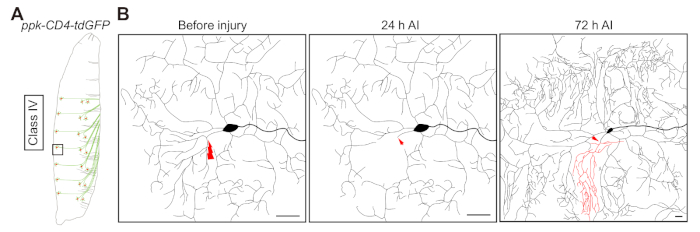
Figure 2: Da neuron dendrite regeneration. (A) Illustrative representation of class IV da neurons. (B) Illustrative representation of dendrite regeneration in class IV da neuron ddaC, labeled by ppk-CD4-tdGFP/+. Laser ablation is targeted to the primary branch point and is conducted at 48 h AEL. At 24 h AI injury transection of the neurite is confirmed, and at 72 h AI regeneration is quantified. Dendrites of ddaC neurons demonstrate substantial regrowth, with new dendritic branches sprouting from the severed stem to tile the vacant space. It is worth noting that new terminal branches are continuously added to the uninjured dendrites at this developmental stage. Scale bar = 20 µm. Please click here to view a larger version of this figure.
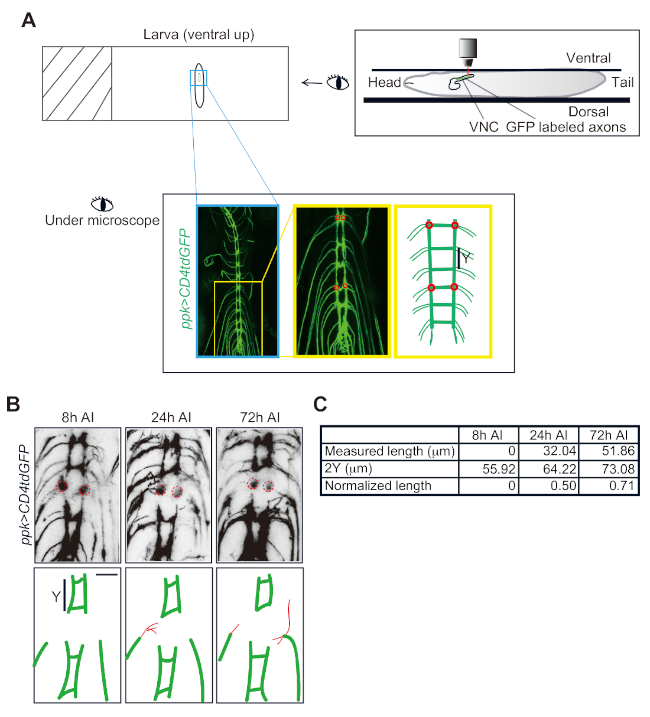
Figure 3: Da neuron axon regeneration in the VNC. (A) Schematic drawing of a Drosophila larva mounted on a slide and imaged under the microscope. Class IV da neuron axons in the VNC visualized in a ppk-CD4tdGFP/+ larva. Two candidate commissure segments are shown in the zoomed-in image and the schematic drawing. Each of them has two injury sites (red circles). (B) Confocal images of one injured segment imaged at 8, 24 and 72 h after injury (AI). Red lines depict the regrowing axons. (C) Measurement and normalization of regrowing axons. Scale bar = 20 µm. Please click here to view a larger version of this figure.
Materials
| Diethyl ether, ACS reagent, anhydrous | Acros Organics | AC615080010 | |
| Halocarbon 27 Oil | Genesee Scientific | 59-133 | |
| Propionic Acid | J.T.Baker | U33007 | |
| Cover Glasses: Rectangles | Fisher Scientific | 12-544-D | 50 mm X 22 mm |
| Zeiss LSM 880 laser scanning microscope | Zeiss | ||
| Zen software | Zeiss | ||
| Chameleon Ultra II | Coherent |

