8.1:
Periodic Classification of the Elements
35,298 Views
•
•
The periodic table arranges atoms based on increasing atomic number so that elements with the same chemical properties recur periodically. When their electron configurations are added to the table, a periodic recurrence of similar electron configurations in the outer shells of these elements is observed. Because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. The outer electrons have the highest energy of the electrons in an atom and are more easily lost or shared than the core electrons. Valence electrons are also the determining factor in some physical properties of the elements.
The horizontal rows are known as periods. Across a period, each consecutive element has an additional proton in the nucleus and an additional electron to the valence shell. The vertical columns are groups. Elements in any one group (or column) have the same number of valence electrons (Figure 1); the alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals beryllium and magnesium each have two, and the halogens fluorine and chlorine each have seven valence electrons. It is the loss, gain, or sharing of valence electrons that defines how elements react. The similarity in chemical properties among elements of the same group occurs because they have the same number of valence electrons.
It is important to remember that the periodic table was developed on the basis of the chemical behavior of the elements, well before any idea of their atomic structure was available. Now the arrangement of the periodic table is understood; elements whose atoms have the same number of valence electrons are in the same group. The colored sections of Figure 1 show the three categories of elements classified by the orbitals being filled.
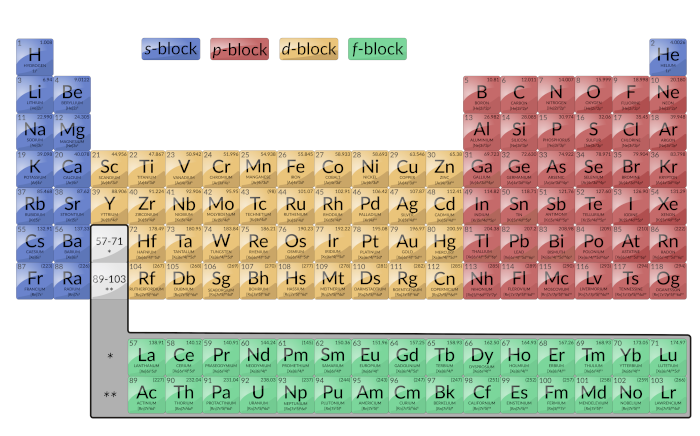
Figure 1: This version of the periodic table shows the configuration of each element. Note that down each group, the configuration is often similar.
Main group elements are sometimes called representative elements. These are the elements in which the last electron added enters an s or a p orbital in the outermost shell, shown in blue and red in Figure 1. This category includes all of the nonmetallic elements, as well as the metalloids and many metals. The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s23d104p1, which contains three valence electrons (underlined). The completely filled d orbitals count as core, not valence, electrons.
The two far-left columns comprise the s-block and the six far-right columns constitute the p-block. The noble gases, which are a part of the p-block, all have eight valence electrons except for helium, which has two. These elements are highly stable and unreactive.
Transition elements or transition metals: These are metallic elements in which the last electron added enters a d orbital. The valence electrons (those added after the last noble gas configuration) in these elements include the ns and (n – 1) d electrons. The official IUPAC definition of transition elements specifies those with partially filled d orbitals. Thus, the elements with completely filled orbitals (Zn, Cd, Hg, as well as Cu, Ag, and Au in Figure 1) are not technically transition elements. However, the term is frequently used to refer to the entire d block (colored yellow in Figure 1).
The d-block consists of 10 columns. The principal quantum number of the d orbitals that fill across each row is equal to the row number minus one. In the fourth row, 3d orbitals fill, in the fifth row, 4d orbitals fill, and so on.
Inner Transition Elements: They are shown in green in Figure 1. The valence shells of the inner transition elements consist of the (n – 2)f, the (n – 1)d, and the ns subshells. Inner transition elements constitute f-block as the last electron enters an f orbital. The principal quantum number of the f orbitals that fill across each row is equal to the row number minus two. In the sixth row, the 4f orbitals fill, and in the seventh row, the 5f orbitals fill. There are two inner transition series:
- The lanthanide series: lanthanide (La) through lutetium (Lu)
- The actinide series: actinide (Ac) through lawrencium (Lr)
Lanthanum and actinium, because of their similarities to the other members of the series, are included and used to name the series, even though they are transition metals with no f electrons.
This text is adapted from OpenStax Chemistry 2e, Section 6.4: Electronic Structure of Atoms.
