3.9:
Experimental Determination of Chemical Formula
32,842 Views
•
•
The elemental makeup of a compound defines its chemical identity, and chemical formulas are the most concise way of representing this elemental makeup. When a compound’s formula is unknown, measuring the mass of its constituent elements is often the first step in determining the formula experimentally.
Determination of Empirical Formulas
The most common approach to determining a compound’s chemical formula is first to measure the masses of its constituent elements. However, chemical formulas represent the relative numbers and not masses of atoms in the substance. Therefore, any experimentally derived data involving mass must be used to obtain the corresponding numbers of atoms in the compound. This is accomplished using molar masses to convert the mass of each element to its number of moles. These molar amounts are used to compute whole-number ratios that can be used to derive the empirical formula of the substance.
Consider a sample of a compound determined to contain 1.71 grams of carbon and 0.287 grams of hydrogen. The corresponding numbers of atoms are 0.142 moles of carbon and 0.284 moles of hydrogen. Thus, this compound may be represented by the formula C0.142H0.284. Per convention, formulas contain whole-number subscripts, which can be achieved by dividing each subscript by the smallest subscript (0.142). The empirical formula for this compound is thus CH2. Subscripts of “1” are not written but rather assumed if no other number is present. This may or not be the compound’s molecular formula; however, additional information is needed to make that determination.
As a second example, a sample of a compound is determined to contain 5.31 grams of chlorine and 8.40 grams of oxygen. The same approach yields a tentative empirical formula of ClO3.5. In this case, dividing by the smallest subscript still leaves a decimal in the empirical formula. To convert this into a whole number, multiply each of the subscripts by two, retaining the same atom ratio and yielding Cl2O7 as the final empirical formula.
Deriving Empirical Formulas from Percent Composition
In instances where the percent composition of a compound is available, it is used to calculate the masses of elements present in the compound. Since the scale for percentages is 100, it is convenient to calculate the mass of elements present in a sample weighing 100 grams. The masses obtained are used to derive the empirical formula.
For example, suppose a gaseous compound contains 27.29% C and 72.71% O. The mass percentages, therefore, are expressed as fractions:


The mass of carbon, 27.29 g, corresponds to 2.272 moles of carbon, and the mass of oxygen, 72.71 g, corresponds to 4.544 moles of oxygen. The representative formula is, therefore, C2.272O4.544. Dividing each subscript with 2.272 provides the empirical formula: CO2.
Derivation of Molecular Formulas
Determining the absolute numbers of atoms that compose a single molecule of a covalent compound requires knowledge of both its empirical formula and its molecular mass or molar mass. These quantities may be determined experimentally by various measurement techniques. Molecular mass, for example, is often derived from the mass spectrum of the compound.
Molecular formulas are derived by comparing the compound’s molar mass or molecular mass to its empirical formula mass. As the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. If the known molar mass of a substance is divided by the empirical formula mass, it yields the number of empirical formula units per molecule (n).
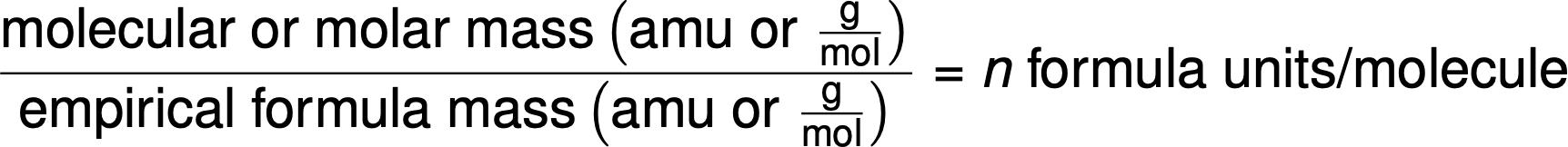
The molecular formula is then obtained by multiplying each subscript in the empirical formula by n, as shown by the generic empirical formula AxBy:
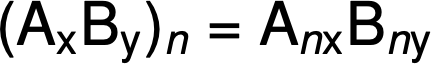
For example, the empirical formula of a covalent compound is determined to be CH2O, and its empirical formula mass is approximately 30 amu. If the compound’s molecular mass is determined to be 180 amu, this indicates that molecules of this compound contain six times the number of atoms represented in the empirical formula.
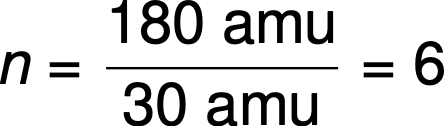
Molecules of this compound are then represented by a molecular formula with subscripts that are six times greater than those in the empirical formula: (CH2O)6 = C6H12O6.
This text is adapted from Openstax, Chemistry 2e, Section 3.2: Determining Empirical and Molecular Formulas.
