Automatically Generated
Determining Total Protein and Bioactive Protein Concentrations in Bovine Colostrum
Summary
Bovine colostrum is both a primary source of nutrients and immunological support for the newborn calf. The understanding of the level of therapeutic proteins (lactoferrin and IgG) is important for the bovine colostrum dosing and standardization for human consumption.
Abstract
Colostrum is a complex biological fluid produced by mammals immediately after parturition. It meets all the nutritional requirements for neonates as a good source of macro- and micronutrients, bioactive peptides, and growth factors. Bovine colostrum is also a potential source of nutrition and bioactive because of its rich protein content that includes immunoglobulin G (IgG) and lactoferrin. However, the level of lactoferrin and IgG in bovine colostrum changes markedly during the lactation period. Therefore, monitoring the concentration of IgG and lactoferrin for the use of bovine colostrum as a protein source is an important question to study. Methods in this article describe how to determine protein content, as well as specific concentrations of lactoferrin and IgG. These methods include the following steps: Isolation of bovine colostrum proteins, Determination of protein concentration via Bicinchoninic acid assay (BCA), Visualization of proteins via SDS-PAGE, Determination of lactoferrin, and IgG concentration using an ELISA Assay.
Introduction
Colostrum is the initial secretion of the mammary gland produced by mammals shortly after parturition. Colostrum is rich in macro- and micronutrients, antimicrobial peptides, and growth factors1,2,3,4. The composition varies gradually over time through the transition to mature milk5,6,7 but most significantly within 24 h after parturition8. The composition of colostrum is also influenced by maternal factors, including age, parity, breed, health, and nutritional status, as well as extrinsic factors, including season, premature parturition, premature lactation, colostral handling factors (pooling colostrum and storage temperature), and induction of parturition9,10,11. Compared with mature milk, colostrum contains less lactose and more fat, protein, peptides, non-protein nitrogen, ash, hormones, growth factors, cytokines, nucleotides, vitamins, and minerals12. Bovine colostrum contains a wide range of proteins, including immunoglobulins, lactoferrin, α-lactalbumin (α-LA), β-lactoglobulin (β-Lg), lactoperoxidase, and several growth factors13. The total protein concentration of bovine colostrum ranges between 11.26 mg/mL and 169.55 mg/mL14. The protein content comprises whey and casein at an average concentration of 124.00 mg/mL and 26.00 mg/mL, respectively15. The whey portion contains three major types of immunoglobulins (Igs) as IgG (85%-90%), IgM (7%), and IgA (5%)16. The major Ig in bovine colostrum is IgG, which provides passive immunity and modulates the adaptive and innate immune systems in the calf17. The initial Ig concentration of the first milking bovine colostrum can range from 20 to 200 mg/mL and decrease to around 0.4-1.0 mg/mL18. The mean IgG concentration is approximately 60 mg/mL and declines steadily to the levels below 1 mg/mL throughout the transition to mature milk19.
Another important bioactive protein in colostrum is lactoferrin, an iron-binding glycoprotein with a concentration of 1.5-5 mg/mL. Properties of lactoferrin include enhancing iron absorption as well as possessing antimicrobial activity20,21, binding lipopolysaccharide, immune-modulation, and stimulating the growth of intestinal epithelial cells and fibroblasts22. Bovine colostrum also contains α-lactalbumin and β-lactoglobulin. These proteins are sources of essential amino acids and also have bactericidal activity23,24,25. The mean α-LA and β-Lg concentrations in colostrum average 2.77 mg/mL2, and 11.5 mg/mL26, respectively. Thereafter, these concentrations decrease to 1-1.5 mg/mL27, and 4.8 mg/mL26 in mature milk. Colostrum also contains a significant amount of lactoperoxidase (mean 22.8 µg/mL) and lysozyme (mean 0.40 µg/mL)26. Lactoperoxidase is a glycoprotein that possesses antimicrobial activity against Gram-positive and negative bacteria28 by producing reactive oxygen species. Lysozyme functions as an antimicrobial agent by cleaving the peptidoglycan component of bacterial cell walls, thereby leading to celldeath29,30.
Due to their properties, IgG and lactoferrin are processed into different food products to fortify infant formulas, food supplements, high-protein preparations for convalescents and sportsmen as well as in pharmacology and cosmetology31,32,33. Bovine colostrum represents an important source of IgG and lactoferrin. However, the composition of these bioactive proteins in bovine colostrum changes markedly during the lactation period. Therefore, monitoring changes in the concentration of these bioactive proteins in colostrum samples used for research and food processing is critical. This study aims to describe the methods for monitoring the concentration and compositions of the total protein, lactoferrin, and IgG in bovine colostrum during the 6 days after calving.
Protocol
Colostrum samples were collected for 6 days after calving in the noon over the period July-August, from 28 Holstein dairy cows from Uluova Milk Trading Company in Çanakkale, Turkey, and deep-frozen. The samples collected on the same day were pooled according to the day of each sample and analyzed for their total protein, lactoferrin, and IgG concentrations. All samples were assayed in duplicate.
1. Sample preparation
- Mix 200 µL of bovine colostrum with 400 µL of dH2O to obtain a diluted sample for the analysis. Dilute all the samples accordingly.
- Centrifuge diluted and undiluted samples at 4 °C, 1000 x g for 30 min.
- Separate the middle phase to an appropriately labeled new tube. Repeat step 1.2. to obtain clear middle phase. Store the whole middle phase and diluted samples at -20 °C, if not used immediately.
- Use the middle phase obtained from diluted samples for BCA, SDS-PAGE, and Lactoferrin assays. Collect the middle phase from undiluted samples for IgG assay.
- Prepare sample dilutions for each sample to ensure that the readings are within the standard curve range. Dilute each middle phase obtained from the diluted samples to 1:300 for BCA assay, and 1:30,000 for Lactoferrin assay, and dilute each middle phase obtained from undiluted samples to 1:400,000 for IgG assay.
NOTE: Dilution factors are determined based on the absorbance value and standard curve.
2. Determine the protein concentration using BCA protein assay kit
- Preparation of standards and reagents
- Use the reagents provided in the commercially available kit (see Table of Materials) to be used for the assay: BCA reagent A, containing sodium carbonate, sodium bicarbonate, bicinchoninic acid, and sodium tartrate in 0.1 M sodium hydroxide. BCA reagent B contains 4% cupric sulfate. Albumin (BSA) standards, contains bovine serum albumin at 2.0 mg/mL in 0.9% saline and 0.05% sodium azide.
- Equilibrate all samples and protein standards to room temperature (RT).
- Prepare sufficient volumes of working reagent (WR) by mixing 50:1 ratio of reagent A:B. 200 µL of WR is required for each sample and standard.
- Prepare diluted albumin (BSA) standards according to the following dilution scheme (Table 1), that presents a working range between 20-2,000 µg/mL final BSA concentration. Use dH2O as diluent.
Table 1: Dilution scheme of BSA standards.
| Vial | Volume of Diluent (µL) | Volume and Source of BSA (µL) | Final BSA Concentration (µg/mL) |
| A | 0 | 300 µl of stock | 2000 |
| B | 125 | 375 µL of stock | 1500 |
| C | 325 | 325 µL of stock | 1000 |
| D | 175 | 175 of vial B diluton | 750 |
| E | 325 | 325 of vial C dilution | 500 |
| F | 325 | 325 of vial E dilution | 250 |
| G | 325 | 325 of vial F dilution | 125 |
| H | 400 | 100 of vial G dilution | 25 |
| I | 400 | 0 | 0 = Blank |
- BCA assay procedure
- Transfer 25 µL of each BCA standard or sample into a 96-well plate. Add 200 µL of the WR to each well-containing standard or sample. Mix the plate thoroughly on a plate shaker for 30 s.
- Cover the plate with a plate sealer and incubate at 37 °C for 30 min. After incubation, allow the reactions to equilibrate to RT for around 10 min. Read each plate at 562 nm using a microplate reader with its associated software.
- Generating standard curve and determining results
- Record the absorbance values for the standards and samples. Please see Table 1 for the dilution series. Subtract absorbance values of the standard blank from each absorbance value of standards and sample. Average the duplicate readings for each standard and sample to estimate total protein concentration.
- Construct a standard curve by plotting the corrected mean absorbance for each standard on the x-axis and concentration on the y-axis. Draw a linear curve with appropriate software capable of the four-parameter curve fit.
- Use the standard curve to determine the concentration of each sample by interpolating its response to the concentration. Multiply with the dilution factor (step 1.5) to obtain the actual concentration of the sample.
3. Visualization of protein using SDS-PAGE assay
- Preparation of sample and solutions
- Prepare stock solutions
- Prepare 10% (w/v) SDS, 1.5 M Tris-HCl pH 8.3, 0.5 M Tris-HCl pH 6.8, 10% (w/v) ammonium persulfate (APS) solution (prepare fresh).
- (w/v) SDS: Weigh 1 g of SDS and add 10 mL of dH2O.
- M Tris-HCl pH 8.8: Weigh 18.15 g of Tris and dissolve with ~60 mL of dH2O. Adjust to pH 8.8 with HCl. Add dH2O to bring the volume to 100 mL.
- M Tris-HCl pH 6.8: Weigh 6.00 g of Tris and dissolve with ~60 mL of dH2O. Adjust to pH 6.8 with HCl. Add dH2O to bring the volume to 100 mL.
- APS: Weigh 15 mg of APS and add 150 µL of dH2O.
CAUTION: Acrylamide and SDS are toxic and harmful. Wear protective gloves and work under a hood.
- Prepare the sample buffer by adding 50 µL of β-Mercaptoethanol into 950 µL of 2x SDS-PAGE sample buffer.
CAUTION: β-mercaptoethanol is toxic if inhaled. Wear protective gloves and work under a hood. - Prepare the running buffer by mixing 100 mL of 10x Tris-glycine SDS Running Buffer with 900 mL of dH2O.
- Prepare the staining solution (45% dH2O, 45% Methanol, 10% glacial acetic acid, 2 g of Coomassie Brilliant Blue R).
- Prepare the destaining solution (50% dH2O, 40% Methanol, 10% glacial acetic acid).
- Prepare stock solutions
- Preparation of gel
- Prepare the electrophoresis unit equipment, including gel cassette, power supplies, electrodes, and cables for the assay. Clean the glass plates with ethanol and assemble the sandwich. Ensure that the lower edges of glass plates and spacers are well aligned.
- Prepare the separating gel mixture containing 3.5 mL of dH2O, 2.4 mL of 40% Acrylamide/Methylene bis Acrylamide, 2 mL of 1.5M Tris-HCl, 100 µL of 10% (w/v) SDS, 80 µL of 10% APS, 8 µL of N,N,N′,N′-Tetramethyl ethylenediamine (TEMED).
CAUTION: TEMED is toxic and/or an irritant. Wear protective gloves and work under a hood. - Pour the separating gel mixture into the gel plates to a level approximately 1-1.5 cm below the top of the shorter plate.
- Layer the top of the separating gel with isopropanol to remove bubbles at the top of the gel and keep the polymerized gel from drying out.
- Pour isopropanol on top of the separating gel after the separating gel has polymerized for at least 15 min.
- Prepare the stacking gel mixture containing 1.92 mL of dH2O, 300 µL of 40% Acrylamide/Methylene bis Acrylamide, 750 µL of 0.5M Tris-HCl, 100 µL of 10% (w/v) SDS, 30 µL of 10% APS, and 3 µL of TEMED.
- Pour the stacking gel solution on top of the separating gel so that gel plates are filled. Insert the comb to the top of the spacers.
- Let the stacking gel polymerize at room temperature for approximately 15 min.
- Running the gel
- Attach the gel to the electrode assembly. Add freshly prepared 1x Tris-glycine SDS Running Buffer to both the chambers of the apparatus.
- Remove the comb.
- Load 5 µL of the ladder (10-250 kDa) and 8 µL of the middle phase of diluted samples into the wells of the gel. Run the gel at 80 V until the dye migrates into the separating gel and increase to 120 V until the dye reaches the bottom of the gel. Turn off the applied power after the dye reaches the bottom of the gel.
- Staining and destaining the gel
- Remove the gel from the apparatus once the run is complete and remove the spacers and glass plates. Place the gel into a small tray.
- Stain the gel by adding staining solution (step 3.1.4) for 30 min with gentle shaking at 55 rpm.
- Pour off the staining solution from the gel. Rinse the gel with a little amount of destaining solution and discard the dye.
- Add a sufficient volume of destaining solution to cover the gel and destain with gentle shaking for ~1 h until the bands are visible.
4. Lactoferrin concentration using a Bovine Lactoferrin ELISA
- Preparation of standards and reagents
- Use the commercially available Bovine LF/LTF/Lactoferrin ELISA Kit for this assay.
- Equilibrate all the samples and standards to RT.
- Prepare sufficient volumes of detection reagent A and B Working Solution that are responsible for binding to the captured antigen.
- Dilute the detection reagents A and B to a ratio of 1:100 using assay diluents A and B, respectively.
- Prepare a 1x working wash buffer by diluting the 30x wash buffer concentrate with dH2O.
- Put a sufficient amount of 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate solution into a sterile microtube.
- Resuspend one tube of lyophilized standard (100 ng/mL) with 0.5 mL of the sample diluent and incubate at RT for 10 min with gentle agitation. Spin the vial to ensure that all of the lyophilized sanple is collected at the bottom.
- Prepare a standard dilution series according to the following dilution scheme (Table 2).
Table 2: Dilution scheme of bovine lactoferrin standards.
| Vial | Volume of Diluent (µL) | Volume and Source of Lf (µL) | Final Lf Concentration (ng/mL) |
| D1 | 0 | 500 µL of stock | 100 |
| D2 | 250 | 250 of vial D1 dilution | 50 |
| D3 | 250 | 250 of vial D2 dilution | 25 |
| D4 | 250 | 250 of vial D3 dilution | 12.5 |
| D5 | 250 | 250 of vial D4 dilution | 6.25 |
| D6 | 250 | 250 of vial D5 dilution | 3,125 |
| D7 | 250 | 250 of vial D6 dilution | 1,563 |
| D8 | 250 | 0 | 0 = Blank |
- Measuring the concentration of Bovine Lactoferrin
- Pipette 100 µL of each lactoferrin standard or sample into the coated 96-well strip plate. Cover the plate with a plate sealer to avoid evaporation. Incubate at 37 °C for 1 h.
- Aspirate the liquid of each well. Add 100 µL of the detection reagent A working solution to each well. Cover with a plate sealer and gently agitate to ensure thorough mixing. Incubate at 37 °C for 1 h.
- Wash three times by adding approximately 350 µL of 1x wash buffer after aspirating the liquid from each well. Allow each wash to sit for 1-2 min before completely aspirating. After the last wash, aspirate to remove any remaining wash buffer, then invert the plate and tap against a clean absorbent paper.
- Add 100 µL of the detection reagent B working solution to each well. Cover with a new plate sealer. Incubate at 37 °C for 30 min. Aspirate the liquid from each well and wash it five times as described in step 4.2.3. Put 90 µL of TMB substrate solution into each well, and cover with a new plate sealer.
- Incubate at 37 °C for 10-20 min away from light. Check the optimal color by monitoring periodically. Observe that the intense blue color in the well includes high concentrate lactoferrin.
- Add 50 µL of stop solution to each well. The color will change from blue to yellow. Measure the absorbance of each well immediately at 450 nm using a microplate reader with its associated software.
NOTE: Tap the plate gently to ensure thorough mixing until the color change is uniform.
- Generating standard curve and determining results
- Follow step 2.3.1 for data generation to estimate lactoferrin concentration.
- Construct a standard curve by plotting the corrected mean absorbance for each standard as described in step 2.3.2 but drawing a polynomial curve with appropriate software capable of the four-parameter curve fit.
- Calculate the lactoferrin content of each sample by interpolating the absorption value onto the generated equation as described in step 2.3.3.
5. IgG concentration determination of samples using Bovine IgG ELISA
- Preparation of standards and reagents
- Use the items required from the ones provided in the Bovine IgG ELISA Kit.
- Equilibrate all the samples and standards to RT.
- Prepare sufficient volumes of working Enzyme-Antibody Conjugate solution by diluting 10 µL of horseradish peroxidase (HRP)-avidin concentrate (100x) with 990 µL of Enzyme-Antibody Conjugate diluent.
- Prepare sufficient volumes of 1x wash buffer by diluting 20x wash buffer concentrate with dH2O.
- Prepare sufficient volumes of the 1x diluent solution by diluting 20x diluent concentrate with dH2O.
- Add 1.0 mL of dH2O to the bovine IgG calibrator and mix gently until dissolved. The final concentration of the calibrator is 123.000 ng/mL.
- Prepare standard dilution series according to the dilution scheme described in Table 3.
Table 3: Dilution scheme of bovine IgG standards.
| Vial | Volume of Diluent (µL) | Volume and Source of IgG (µL) | Final IgG Concentration (ng/mL) |
| D1 | 900 | 100 µL of stock | 12300 |
| D2 | 900 | 100 of vial D1 dilution | 1230 |
| D3 | 178 | 122 of vial D2 dilution | 500 |
| D4 | 150 | 50 of vial D3 dilution | 250 |
| D5 | 150 | 150 of vial D4 dilution | 125 |
| D6 | 100 | 100 of vial D5 dilution | 62.5 |
| D7 | 100 | 100 of vial D6 dilution | 31.25 |
| D8 | 100 | 100 of vial D7 dilution | 15,625 |
| D9 | 100 | 100 of vial D8 dilution | 7,813 |
| D10 | 100 | 0 | 0 = Blank |
- Bovine IgG ELISA assay procedure
- Pipette 100 µL of each IgG standard or sample into the coated 96-well strip plate. Cover the plate with a plate sealer and incubate at RT for 30 min. Aspirate the liquid from each well.
- Wash four times by filling wells with 1x wash buffer and aspirate. After the last wash, aspirate to remove any residual wash buffer, then invert the plate and tap against clean absorbent paper. Add 100 µL of appropriately diluted Enzyme-Antibody Conjugate to each well. Cover with a plate sealer and gently agitate to ensure thorough mixing.
- Incubate at RT for 10 min. Wash and remove residual wash buffer from the wells as described in step 5.2.2. Add 100 µL of TMB substrate to each well; cover with a new plate sealer.
- Incubate at RT for precisely 10 min away from light. Stop the reaction by adding 100 µL of stop solution to each well. Read each plate at 450 nm using a microplate reader with its associated software.
- Generating standard curve and determining results
- Follow step 2.3.1 for the data edition to estimate IgG concentration.
- Construct a standard curve by plotting the concentration on the x-axis and the corrected mean absorbance for each standard on the y-axis. Draw a polynomial curve with appropriate software capable of the four-parameter curve fit.
- Calculate the IgG content of each sample by interpolating the absorption value onto the generated equation as described in step 2.3.3.
Representative Results
Following the protocol, the bovine colostrum samples were analyzed to determine protein, lactoferrin, and IgG concentration. The results of protein, lactoferrin, and IgG analyses of bovine colostrum are shown in Table 4.
Table 4: Concentration of protein, lactoferrin, and IgG of bovine colostrum.
| Bovine Colostrum | |||
| Days | Protein (mg/mL) | Lactoferrin (mg/mL) | IgG (mg/mL) |
| 0 | 154.85 ± 43.88 | 1.72 ± 0.21 | 78.30 ± 29.01 |
| 1 | 37.75 ± 16.77 | 0.33 ± 0.04 | 25.22 ± 16.69 |
| 2 | 23.57 ± 1.42 | 0.23 ± 0.04 | 10.24 ± 4.76 |
| 3 | 18.42 ± 5.13 | 0.15 ± 0.12 | 5.73 ± 6.07 |
| 4 | 19.28 ± 1.69 | 0.10 ± 0.11 | 1.24 ± 0.46 |
| 5 | 16.93 ± 0.93 | 0.13 ± 0.03 | 1.60 ± 1.29 |
| 6 | 15.72 ± 0.81 | 0.12 ± 0.06 | 0.42 ± 0.09 |
The protein concentrations of the samples were determined with the BCA assay, which is a Copper-based colorimetric method for quantification of total protein in a sample. In this method, Cu+2 is reduced to Cu+ by an alkaline medium, and Cu+ reacts with the BCA resulting in intense purple color. This method is robust and highly sensitive over a broad range of protein concentrations and protein compositions. In this method, the samples were put into the wells, and a working reagent was added to each well and incubated at 37 °C for 30 min. After incubation, the plate was cooled to RT, and absorbance was measured at 562 nm on a plate reader.The changes in protein concentrations of bovine colostrum from 28 bovines collected at different time points are shown in Figure 1. The mean concentration of protein was approximately 41.00 mg/mL ranging from 154.85 to 15.72 mg/mL. Similar results were reported by Zhang et al. (2015)14. These results indicated an approximately 10-fold decrease in the protein concentrations from day 0 to day 6 and the rate of change was especially high within 24 h.
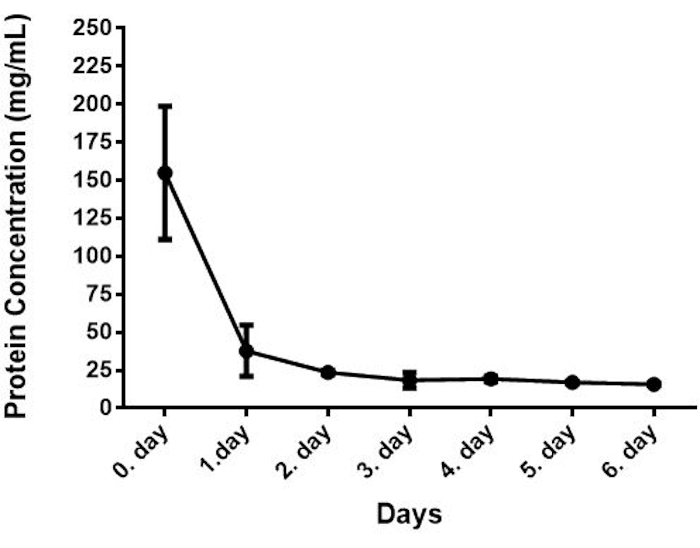
Figure 1: Protein concentration of bovine colostrum (n=28). Please click here to view a larger version of this figure.
Qualitative analysis of protein molecular weight and semi-quantitative analysis of protein aggregates and fragments of bovine colostrum was performed using SDS-PAGE (step 3). In this method, each protein is separated based on its molecular mass. The protein fragments of bovine colostrum are shown in Figure 2, as shown in different studies34,35,36.
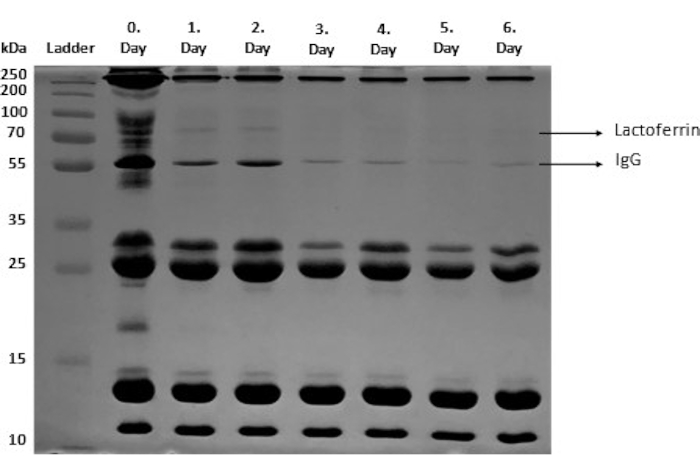
Figure 2: SDS-PAGE of bovine colostrum samples. IgG and Lactoferrin bands are shown. Please click here to view a larger version of this figure.
The lactoferrin and IgG concentrations were determined by sandwich ELISA, which measures the antigen concentration in an unknown sample. In this ELISA model, the target antigen is detected via anchoring between two antibodies37. This type of ELISA has more advantages such as high detection sensitivity, high specificity, and fast and accurate detection of antigen concentration in an unknown sample because of the use of two antibodies38. In the lactoferrin detection method, samples were added to the wells that are pre-coated with a lactoferrin-specific capture antibody, and target antigens were bound to capture the antibody. Unbound samples were washed away, and a biotin-conjugated detection antibody was added to each well to bind to the captured antigen. Unbound detection antibodies were washed away, and HRP conjugate was added to bind to the biotin and then unbound Avidin-HRP conjugate was washed away. TMB substrate was added to each well to result in color development through reacting with the HRP enzyme. The reaction was terminated by adding the sulfuric acid stop reaction (step 4)39,40,41. The amount of sample lactoferrin is proportional to the intensity of coloration. The concentration of lactoferrin in the first bovine colostrum was 1.72 mg/mL, which decreased to the value of 0.12 mg/mL after 6 days of parturition as shown in Figure 3. The concentrations of lactoferrin detected here are in line with previous reports1,42.
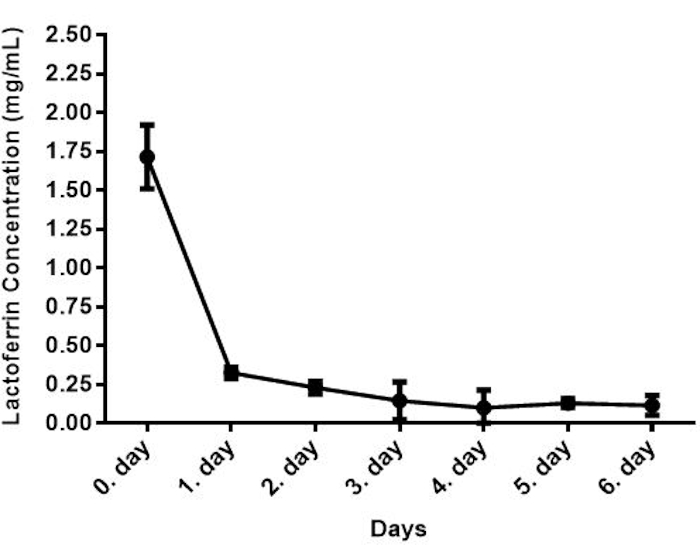
Figure 3: Lactoferrin concentration of bovine colostrum (n=28). Please click here to view a larger version of this figure.
In the IgG detection method, samples were added to the wells that were pre-coated with IgG-specific antibody, incubated at RT for 30 min, and then removed the contents of the wells. Anti-IgG-HRP conjugated detector antibody was added into the wells, incubated for 10 min, and the unbound conjugate was washed away. The TMB substrate was added, which is catalyzed by HRP generating a blue color product to produce the enzymatic reaction. Formation of yellow color was obtained through the addition of an acidic stop solution. The amount of sample IgG is proportional to the intensity of yellow coloration39,40,41. The concentration of IgG concentration in the bovine colostrum samples is shown in Figure 4. The IgG concentration was found to be 78.30 mg/mL for the first day after parturition. A similar result was reported previously3,43,44. This concentration is within the range reported in the literature42,45,46,47,48,49. The concentration of IgG was the highest in the first colostrum and thereafter rapidly decreased.
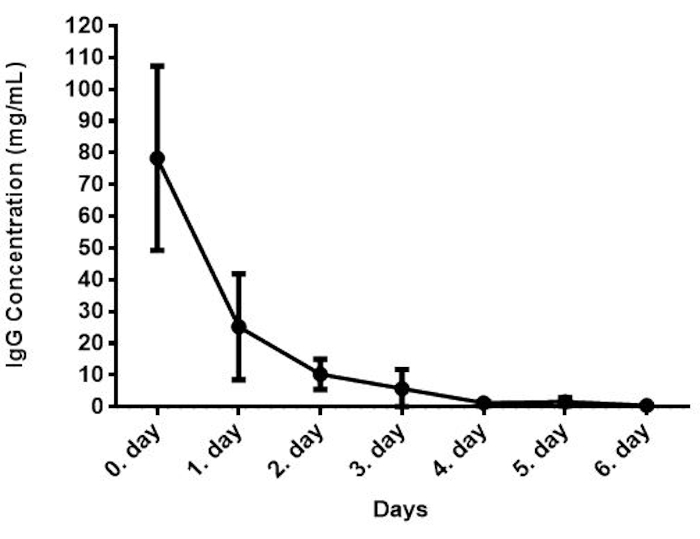
Figure 4: IgG concentration of bovine colostrum (n=28). Please click here to view a larger version of this figure.
Discussion
This study provides information about considerable changes in the protein, lactoferrin, and IgG concentrations in colostrum throughout the transition to mature milk. Detection of changes in the lactoferrin and IgG concentration was carried out by sandwich ELISA, and total protein concentration was analyzed by the BCA assay. Results indicate that early colostrum has the highest protein, lactoferrin, and IgG concentration, that subsequently decreased over the next 3 days. Accurate measurements of these proteins are relevant for the use of colostrum in the production of dairy foods such as yogurt50,51, milk drinks, and butter52, ice cream53, and fermented milks54. Bioactive proteins from bovine colostrum (e.g., lactoferrin, lysozyme, and Igs) are also utilized in the pharmaceutical industry55. Here, we showed that the composition of bovine colostrum is changing substantially over time. Thus, efficient and robust detection methods are critical to assess bovine colostrum and its derived bioactive components as supplementary ingredients in functional foods and the pharmaceutical industry.
The BCA assay and ELISA are common techniques in molecular biology due to their high sensitivity of detection56. BCA is simpler and faster with its single process compared to the classical Lowry assay that has two steps57. BCA is advantageous over most dye-binding methods due to its robustness and reproducibility over a wide range of protein concentrations and compositions. Notably, while some detergents and denaturing agents such as urea and guanidinium chloride do not affect BCA, reducing sugars may have a negative effect58. Another advantage of the BCA assay is the possibility of assaying whole-cell lysates, affinity-column fractions, purified protein samples; this is a suitable method for industrial applications. One main disadvantage of this method is that incubation time is a critical step. Because the BCA method is not a true end-point method, the color development continues through incubation. Therefore, increasing incubation time can cause an increase in the net absorbance, thus decreasing the minimum detection level and the working range of the protocol58. Additionally, the presence of the cysteine, tyrosine, and tryptophan residues in the solution may affect the reaction and interfere with the results58.
Bioactive multifunctional proteins lactoferrin and IgG have been detected and determined by various methods, including aptasensor, electrophoresis, chromatography radial immunodiffusion, and immunoassay according to their properties59. Among these methods, ELISA stands out with the advantages of being a simple procedure with high sensitivity and selectivity. Compared to other assays, ELISAs are compatible with high throughput testing, and most reagents are affordable, safe, and eco-friendly60. The ELISA method is widely used for the detection and quantification of low concentration components of serum, plasma, and other biological fluids as peptides, proteins, antibodies, hormones, drugs, a range of metabolites, and allergens. The Sandwich ELISA, containing two antibodies, detects different epitopes on the same target antigen, thus presenting high specificity sensitivity and a wider working range. It is also suitable for the accurate detection of antigens in unknown samples, and the antigen does not need to be purified beforehand. It can be utilized successfully for the determination of antigens present in low abundance, thus making it an ideal method for obtaining repeatable and precise results when working on complex substrates61.
To obtain the best results, ELISA methods must be optimized. The aims of the optimization in assay development are both achieving a high signal-to-noise ratio and maintaining optimal responses. One of the essential steps for optimizing ELISA is washing. A thorough washing procedure is necessary to reduce background signal related to unbound, conjugated antibody and increase the assay's signal-to-noise ratio. Insufficient washing can result in poor precision and falsely elevated absorbance and thus poor results. Assay timing and the incubation steps also play a key role in this assay. The interval between adding samples from the first to last wells should be minimized to prevent evaporation and drying out the wells. The other significant factor that impacts the ELISA is sample preparation. The samples should be prepared in different concentrations considering the detection limit of the substrate. Insufficient dilution can cause underestimation of the concentration, while overestimation can occur in excessive dilutions. Additionally, dilution optimization must be performed to ensure that the results fall within the linear portion of the standard curve62,63.
Bovine colostrum is a high nutritional value fluid for a variety of food and functional applications. Components of colostrum have shown promise in various aspects of human health, including maintenance of gastrointestinal integrity64, preventing and resolving microbial infections5, reducing the number of upper respiratory tract infections and diarrheal episodes in children65,66 in addition to enhancing performance and recovery for athletes67,68. Therefore, utilizing bovine colostrum or its bioactive components as a supplement has attracted considerable attention in different research and industrial fields. Detection methods are critical to accurately determine the level of the bioactive components of bovine colostrum. Here, we showed the application of the two commonly used and well-validated, widely used methods in molecular biology (ELISA and the BCA assay) for the detection of bioactive proteins of interest in colostrum. These methods are cost-effective, sensitive, accurate, and robust, and, importantly, are adaptable to high throughput applications, making them ideal candidates for both academic research and industrial applications.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study is supported by Uluova Süt Ticaret A.Ş (Uluova Milk Trading Co.). RMD and BMH are employees of Evolve BioSystems, a company focused on restoring the infant microbiome.
Materials
| 10X Running Buffer (Tris-Glycine-SDS) | ClearBand | TGS10 | SDS-Page analysis |
| 2-mercaptoethanol | gibco | 31350-010 | SDS-Page analysis |
| Acetic Acid GLACIAL | Isolab | 901,013,2500 | SDS-Page analysis |
| Bovine IgG ELISA Kit | Aviva Systems Biology | OKIA00005 | Determination of IgG concentration |
| Bovine LF / LTF / Lactoferrin ELISA Kit | LSBio Lifespan Biosciences | LS-F4884 | Determinaton of lactoferrin concentration |
| Coomassie Brillant Blue R 250 | amresco | 0472-25G | SDS-Page analysis |
| Hydrochloric Acid Fuming 37% | Isolab | 932,103,2501 | SDS-Page analysis |
| Isopropanol | Isolab | 961,023,2500 | SDS-Page analysis |
| Laemmli Sample Buffer (2X) | ClearBand | LSB-2x | SDS-Page analysis |
| Methanol | Isolab | 947,046,2500 | SDS-Page analysis |
| PageRuler Plus Prestained Protein Ladder 10 to 250 | Thermo Scientific | 26619 | SDS-Page analysis |
| Pierce BCA Protein Assay Kit | Thermo Scientific | 23225 | Determination of protein concentration |
| Sodium dodecyl sulfate (SDS) | BioShop | SDS001.500 | SDS-Page analysis |
| SureCast Acrylamide Solution 40% (w/v) | Invitrogen | HC2040 | SDS-Page analysis |
| SureCast Ammonium persulfate (APS) | Thermo Scientific | 17874 | SDS-Page analysis |
| SureCast Tetramethylethylenediamine (TEMED) | Invitrogen | HC2006 | SDS-Page analysis |
| TECAN Infinite M200 Plate Reader | Tecan | 30035094 | Measurement of absorbance |
| Tris base | BioShop | TRS001.1 | SDS-Page analysis |
References
- Kehoe, S. I., Jayarao, B. M., Heinrichs, A. J. A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. Journal of Dairy Science. 90 (9), 4108-4116 (2007).
- Levieux, D., Ollier, A. Bovine immunoglobulin G, β-lactoglobulin, α-lactalbumin and serum albumin in colostrum and milk during the early post partum period. Journal of Dairy Research. 66 (3), 421-430 (1999).
- Elfstrand, L., Lindmark-Månsson, H., Paulsson, M., Nyberg, L., Åkesson, B. Immunoglobulins, growth factors and growth hormone in bovine colostrum and the effects of processing. International Dairy Journal. 12 (11), 879-887 (2002).
- Strekozov, N. I., Motova, E. N., Fedorov, Y. N. Evaluation of the chemical composition and immunological properties of colostrum of cows’ first milk yield. Russian Agricultural Sciences. 34 (4), 259-260 (2008).
- Playford, R. J., Weiser, M. J. Bovine colostrum: Its constituents and uses. Nutrients. 13 (1), 265 (2021).
- Godhia, M., Patel, N. Colostrum – Its composition, benefits as a nutraceutical: A review. Current Research in Nutrition and Food Science Journal. 1 (1), 37-47 (2013).
- Nakamura, T., et al. Concentrations of sialyloligosaccharides in bovine colostrum and milk during the prepartum and early lactation. Journal of Dairy Science. 86 (4), 1315-1320 (2003).
- Arain, H. H., Khaskheli, M., Arain, M. A., Soomro, A. H., Nizamani, A. H. Heat stability and quality characteristics of postpartum buffalo milk. Pakistan Journal of Nutrition. 7 (2), 303-307 (2008).
- Maunsell, F. P., et al. Effects of mastitis on the volume and composition of colostrum produced by Holstein cows. Journal of Dairy Science. 81 (5), 1291-1299 (1998).
- Tittle, D. J. Factors affecting colostrum quality. Cattle Practice. 10 (2), 131-136 (2002).
- Zarcula, S., et al. Influence of breed, parity and food intake on chemical composition of first colostrum in cow. Animal Science and Biotechnology. 43 (1), 43 (2010).
- McGrath, B. A., Fox, P. F., McSweeney, P. L. H., Kelly, A. L. Composition and properties of bovine colostrum: a review. Dairy Science and Technology. 96 (2), 133-158 (2016).
- Bastian, S. E. P., Dunbar, A. J., Priebe, I. K., Owens, P. C., Goddard, C. Measurement of betacellulin levels in bovine serum, colostrum and milk. Journal of Endocrinology. 168 (1), 203-212 (2001).
- Zhang, L., et al. Bovine milk proteome in the first 9 days: Protein interactions in maturation of the immune and digestive system of the newborn. PloS One. 10 (2), 0116710 (2015).
- Godden, S. Colostrum management for dairy calves. The Veterinary clinics of North America. Food Animal Practice. 24 (1), 19-39 (2008).
- Larson, B. L., Heary, H. L., Devery, J. E. Immunoglobulin production and transport by the mammary gland. Journal of Dairy Science. 63 (4), 665-671 (1980).
- Ulfman, L. H., Leusen, J. H. W., Savelkoul, H. F. J., Warner, J. O., van Neerven, R. J. J. Effects of bovine immunoglobulins on immune function, allergy, and infection. Frontiers in Nutrition. 5, 52 (2018).
- El-Loly, M. M. Bovine milk immunoglobulins in relation to human health. International Journal of Dairy Science. 2 (3), 183-195 (2007).
- Korhonen, H., Marnila, P., Gill, H. S. Milk immunoglobulins and complement factors. British Journal of Nutrition. 84 (1), 75-80 (2000).
- Arnold, R. R., Brewer, M., Gauthier, J. J. Bactericidal activity of human lactoferrin: Sensitivity of a variety of microorganisms. Infection and Immunity. 28 (3), 893-898 (1980).
- Aisen, P., Listowsky, I. Iron transport and storage proteins. Annual Review of Biochemistry. 49 (1), 357-393 (1980).
- Zhao, X., et al. The in vitro protective role of bovine lactoferrin on intestinal epithelial barrier. Molecules. 24 (1), 148 (2019).
- Chatterton, D. E. W., Smithers, G., Roupas, P., Brodkorb, A. Bioactivity of β-lactoglobulin and α-lactalbumin-Technological implications for processing. International Dairy Journal. 16 (11), 1229-1240 (2006).
- Pellegrini, A., Dettling, C., Thomas, U., Hunziker, P. Isolation and characterization of four bactericidal domains in the bovine β-lactoglobulin. Biochimica et Biophysica Acta (BBA) – General Subjects. 1526 (2), 131-140 (2001).
- Brück, W. M., et al. rRNA probes used to quantify the effects of glycomacropeptide and α-lactalbumin supplementation on the predominant groups of intestinal bacteria of infant rhesus monkeys challenged with enteropathogenic Escherichia coli. Journal of Pediatric Gastroenterology and Nutrition. 37 (3), 273-280 (2003).
- Indyk, H. E., Hart, S., Meerkerk, T., Gill, B. D., Woollard, D. C. The β-lactoglobulin content of bovine milk: Development and application of a biosensor immunoassay. International Dairy Journal. 73, 68-73 (2017).
- Swaisgood, H. E. Protein and amino acid composition of bovine milk. Handbook of Milk Composition. , 464-468 (1995).
- Seifu, E., Buys, E. M., Donkin, E. F. Significance of the lactoperoxidase system in the dairy industry and its potential applications: A review. Trends in Food Science and Technology. 16 (4), 137-154 (2005).
- Wheeler, T. T., Hodgkinson, A. J., Prosser, C. G., Davis, S. R. Immune components of colostrum and milk-A historical perspective. Journal of Mammary Gland Biology and Neoplasia. 12 (4), 237-247 (2007).
- Clare, D., Catignani, G., Swaisgood, H. Biodefense properties of milk: The role of antimicrobial proteins and peptides. Current Pharmaceutical Design. 9 (16), 1239-1255 (2003).
- Mehra, R., Marnila, P., Korhonen, H. Milk immunoglobulins for health promotion. International Dairy Journal. 16 (11), 1262-1271 (2006).
- Gapper, L. W., Copestake, D. E. J., Otter, D. E., Indyk, H. E. Analysis of bovine immunoglobulin G in milk, colostrum and dietary supplements: a review. Analytical and Bioanalytical Chemistry. 389 (1), 93-109 (2007).
- Mettler, A. E. Utilization of whey by-products for infant feeding. International Journal of Dairy Technology. 33 (2), 67-72 (1980).
- Zenker, H. E., Raupbach, J., Boeren, S., Wichers, H. J., Hettinga, K. A. The effect of low vs. high temperature dry heating on solubility and digestibility of cow’s milk protein. Food Hydrocolloids. 109, 106098 (2020).
- Costa, F. F., et al. Microfluidic chip electrophoresis investigation of major milk proteins: Study of buffer effects and quantitative approaching. Analytical Methods. 6 (6), 1666-1673 (2014).
- Lönnerdal, B., Du, X., Jiang, R. Biological activities of commercial bovine lactoferrin sources. Biochemistry and Cell Biology. 99 (1), 35-46 (2021).
- Belanger, L., Sylvestre, C., Dufour, D. Enzyme-linked immunoassay for alpha-fetoprotein by competitive and sandwich procedures. Clinica Chimica Acta. 48 (1), 15-18 (1973).
- Sakamoto, S., et al. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. Journal of Natural Medicines. 72 (1), 32-42 (2018).
- Engvall, E. The ELISA, enzyme-linked immunosorbent assay. Clinical Chemistry. 56 (2), 319-320 (2010).
- Kohl, T. O., Ascoli, C. A. Immunometric double-antibody sandwich enzyme-linked immunosorbent assay. Cold Spring Harbor Protocols. 2017 (6), (2017).
- Shah, K., Maghsoudlou, P. Enzyme-linked immunosorbent assay (ELISA): the basics. British Journal of Hospital Medicine. 77 (7), 98-101 (2016).
- Abd El-Fattah, A. M., Abd Rabo, F. H. R., EL-Dieb, S. M., El-Kashef, H. A. Changes in composition of colostrum of Egyptian buffaloes and Holstein cows. BMC Veterinary Research. 8 (1), 19 (2012).
- Newby, T. J., Stokes, C. R., Bourne, F. J. Immunological activities of milk. Veterinary Immunology and Immunopathology. 3 (1-2), 67-94 (1982).
- Chigerwe, M., et al. Comparison of four methods to assess colostral IgG concentration in dairy cows. Journal of the American Veterinary Medical Association. 233 (5), 761-766 (2008).
- Foley, J. A., Otterby, D. E. Availability, storage, treatment, composition, and feeding value of surplus colostrum: A review. Journal of Dairy Science. 61 (8), 1033-1060 (1978).
- Mechor, G. D., Gröhn, Y. T., McDowell, L. R., Van Saun, R. J. Specific gravity of bovine colostrum immunoglobulins as affected by temperature and colostrum components. Journal of Dairy Science. 75 (11), 3131-3135 (1992).
- Pritchett, L. C., Gay, C. C., Besser, T. E., Hancock, D. D. Management and production factors influencing immunoglobulin G1 concentration in colostrum from Holstein cows. Journal of Dairy Science. 74 (7), 2336-2341 (1991).
- Quigley, J. D., Martin, K. R., Dowlen, H. H. Concentrations of trypsin inhibitor and immunoglobulins in colostrum of Jersey cows. Journal of Dairy Science. 78 (7), 1573-1577 (1995).
- Bielmann, V., et al. An evaluation of Brix refractometry instruments for measurement of colostrum quality in dairy cattle. Journal of Dairy Science. 93 (8), 3713-3721 (2010).
- A Ayar, A., Sıçramaz, H., Çetin, I. The effect of bovine colostrum on the lactic flora of yogurt and kefir. JSM Biotechnology and Biomedical Engineering. 3, 3-8 (2016).
- Sobaih, A., Zaki, D. A. Production of novel functional yoghurt fortified with bovine colostrum and date syrup for children. Alexandria Science Exchange Journal. 39, 651-662 (2018).
- Saalfeld, M. H., et al. Colostro: a redescoberta de um alimento saudável, nutritivo e com potencial probiótico. Agroecologia e Desenvolvimento Rural Sustentável. 5 (2), 18-24 (2012).
- Mouton, E., Aryana, K. J. Influence of colostrum on the characteristics of ice cream. Food and Nutrition Sciences. 06 (05), 480-484 (2015).
- Nazir, T., Pal, M. A., Manzoor, A. Effect of admixing varying levels of whole milk to the colostrum on the sensory quality of fermented colostrum product. International Journal of Advanced Research in Science, Engineering and Technology. 7 (4), 156-161 (2018).
- Korhonen, H. J. Bioactive milk proteins, peptides and lipids and other functional components derived from milk and bovine colostrum. Functional Foods. , 471-511 (2011).
- Cortés-Ríos, J., et al. Protein quantification by bicinchoninic acid (BCA) assay follows complex kinetics and can be performed at short incubation times. Analytical Biochemistry. 608, 113904 (2020).
- Johnson, M. Protein quantitation. Materials and Methods. 2, 115 (2012).
- Walker, J. M. The Bicinchoninic Acid (BCA) assay for protein quantitation. The Protein Protocols Handbook. , 11-15 (2009).
- Wang, R., et al. Sensitive immunoassays based on specific monoclonal IgG for determination of bovine lactoferrin in cow milk samples. Food Chemistry. 338, 127820 (2021).
- Kazemi, M. G., Feizy, J. Overview of the important of ELISA technique and application in food industry. Analyzing Microbes. 4 (4), 19-25 (2020).
- Verma, J., Saxena, S., Babu, S. G. ELISA-based identification and detection of microbes. Analyzing Microbes. , 169-186 (2013).
- Minic, R., Zivkovic, I. Optimization, validation and standardization of ELISA. Norovirus. , (2020).
- Drijvers, J. M., Awan, I. M., Perugino, C. A., Rosenberg, I. M., Pillai, S. The enzyme-linked immunosorbent assay. Basic Science Methods for Clinical Researchers. , 119-133 (2017).
- Walker, A. Breast milk as the gold standard for protective nutrients. The Journal of Pediatrics. 156 (2), 3-7 (2010).
- Patel, K., Rana, R. Pedimune in recurrent respiratory infection and diarrhoea-The Indian experience-The PRIDE study. The Indian Journal of Pediatrics. 73 (7), 585-591 (2006).
- Saad, K., et al. Effects of bovine colostrum on recurrent respiratory tract infections and diarrhea in children. Medicine. 95 (37), 4560 (2016).
- Buckley, J. D., Brinkworth, G. D., Abbott, M. J. Effect of bovine colostrum on anaerobic exercise performance and plasma insulin-like growth factor I. Journal of Sports Sciences. 21 (7), 577-588 (2003).
- Kotsis, Y., et al. A low-dose, 6-week bovine colostrum supplementation maintains performance and attenuates inflammatory indices following a Loughborough Intermittent Shuttle Test in soccer players. European Journal of Nutrition. 57 (3), 1181-1195 (2018).

.
