Functional Isolation of Single Motor Units of Rat Medial Gastrocnemius Muscle
Summary
This method allows the recording of the force of twitch and tetanic contractions and action potentials in three types of motor units in the rat medial gastrocnemius muscle. The functional isolation of a single motor unit is induced by electrical stimulation of the axon.
Abstract
This work outlines functional isolation of motor units (MUs), a standard electrophysiological method for determining characteristics of motor units in hindlimb muscles (such as the medial gastrocnemius, soleus, or plantaris muscle) in experimental rats. A crucial element of the method is the application of electrical stimuli delivered to a motor axon isolated from the ventral root. The stimuli may be delivered at constant or variable inter-pulse intervals. This method is suitable for experiments on animals at varying stages of maturity (young, adult or old). Moreover, this protocol can be used in experiments studying variability and plasticity of motor units evoked by a large spectrum of interventions. The results of these experiments may both augment basic knowledge in muscle physiology and be translated into practical applications. This procedure focuses on the surgical preparation for the recording and stimulation of MUs, with an emphasis on the necessary steps to achieve preparation stability and reproducibility of results.
Introduction
Motor units (MUs) are the smallest functional units of skeletal muscles. Therefore, understanding their function, plasticity and contractile properties, as well as the mechanisms of their force regulation, is crucial for progress in muscle physiology. The basic contractile properties of MUs and the proportions of their physiological types have been documented for numerous muscles, predominantly the hindlimb muscles in experimental animals. However, both the plasticity of MU properties and the mechanisms of MU force regulation are still not fully understood.
The principle of the described method is extensive denervation of the hindlimb muscles except the investigated one and laminectomy at the lumbar vertebrae in order to prepare thin ventral rootlets, each one containing a single "functional" motor axon, stimulated electrically to record the force and action potential of the MU. Using the technique described in this paper, it is possible to isolate more than half of the MUs of the medial gastrocnemius muscle in a successful experiment. The rat medial gastrocnemius is composed of on average 52 MUs (females) or 57 MUs (males) of three physiological types: S (slow), FR (fast resistant) and FF (fast fatigable)1,2, and have variable contractile properties3. For experiments comparing mean values for MUs in the control and experimental groups, isolation and recording of 10-30 MUs for each of these groups are necessary. Critically, individual MUs may be accessible for stimulation for time periods exceeding one hour. Moreover, since this technique allows for recording both MU force and action potentials, this method is suitable for studying phenomena associated with force production, assessing the effect of fatigue, and observing the relationship between the force and action potentials.
Previous studies have confirmed that MU contractile properties are plastic and may be modulated by numerous interventions. Experiments using the technique described here have been performed on rat medial gastrocnemius4 or other hindlimb muscles of the rat5,6 as well as on cat muscles7, using a similar method of single MU isolation. Another series of experiments using trains of stimuli delivered at variable inter-pulse intervals provided observations concerning motor control processes, and the results in general turn attention to the history of stimulation, including considerable effects of a shift in time scale of even one stimulus, crucial for force production8,9.
MUs may also be studied using alternative methods. First, one method is direct stimulation of motoneurons. Burke used intracellular stimulation of motoneurons in cat medial gastrocnemius and soleus with glass microelectrodes used in parallel to determine the electrophysiological properties of these neurons1,10. Other methods have been proposed to study MUs in human muscles, which require considerably lower intervention. For all these methods, the stimulating and recordings electrodes are inserted into the muscle or nerve, and force is recorded from the finger or from the foot. The first of these methods was used to study MUs in the first dorsal interosseous muscle. For this muscle, contracting with a low force, in the electromyogram recorded with the needle electrode inserted into the muscle the action potentials of only one active motor unit were identified. Then the fragments of a muscle force recorded in parallel and following each action potential were averaged (spike-triggered averaging). This method enables extraction of the force of one motor unit from the muscle force recording11. However, the methodological weakness of this procedure is that no single twitch force but rather fragments of tetanic contractions were averaged. Human MUs may also be studied using the second method of intramuscular electrical microstimulation using an electrode inserted into the muscle12, which stimulates a fragment of an axonal tree, leading to activation of a single motor unit. The third method is microstimulation with an electrode inserted into the nerve. When the electrode activates only one motor axon in the nerve, only one motor unit contracts13. These last methods have some limitations, including stability and quality of the recording, ethical restrictions and access to the experimental material. This protocol has been extensively used in cats in the 70's and 80's14.
Protocol
All procedures need to be approved by the local ethics committee and adhered to the European Union guidelines on animal care as well as the national law on the protection of animals.
NOTE: Each experimenter involved in this procedure must be trained in basic surgical procedures and has to obtain a valid license for performing animal experiments.
1. Anesthesia
- Anesthetize the rat with an intraperitoneal injection of sodium pentobarbital (an initial dose of 60 mg·kg-1).
- After approximately 5 min, check the depth of anesthesia by pinching the rat’s ear or forelimb with blunt forceps. Go to the next steps of the protocol only when no reflex action is observed.
- During the surgery, check the animal for reflex actions every 10-15 minutes and supplement anesthesia if the animal responds to a pinch with movement (usually, 10 mg·kg-1·h-1 sodium pentobarbital, IP).
2. Surgery
- Prepare the animal for the surgical procedure by shaving the fur over the left hindlimb from the heel to the hip (the first segment, muscle and nerve isolation), the right hindlimb from the heel to the hip (the second segment, ground electrode), and the backside from the tail to the thoracic segments (the third segment, laminectomy). Antiseptic is not necessary because of the acute nature of the experiment.
- Place the rat on its abdomen on a heating pad (37 °C ± 1 °C.)
- Laminectomy
- Using sharp-blunt scissors, cut the skin along the spinal column from the sacrum up to the thoracic vertebrae.
- Separate the skin from the underlying muscles.
- Using blunt tip scissors, cut out the longissimus muscle on both sides of the sacrum and lumbar spinous processes.
- Identify the S1 vertebra as the lowest segment. Using sharp-blunt scissors, cut and remove the spinous processes from L6 to L2 vertebrae. Next, using fine rongeurs, remove the transverse processes L6-L2 and perform a laminectomy over L6 – L2 segments (first transverse processes, then lamina, begin with L6 vertebral segment) to expose the lumbar segments of the spinal cord covered by the dura mater. Be careful not to cut the sacral bone and L1 spinous process, which will be used as a fixation point for animal immobilization.
- Using sharp scissors, cut the spinal cord (its caudal fragment) and the dorsal and ventral roots at L2 vertebrae segment level, at the upper border of laminectomy. Place small pieces of dried gel foam to stop bleeding. Next, place a thin, saline-soaked cotton wool over the exposed spinal cord segments.
- Isolation of medial gastrocnemius muscle and its nerve
- Using sharp-blunt scissors, make a longitudinal cut on the posterior side of the left hind limb, from the Achilles tendon to the hip.
- Grasp the skin with the forceps and separate the skin from the underlying muscles on both sides of the incision.
- Locate the popliteal fossa at the back of the knee joint, which is covered by the biceps femoris muscle. Using scissors, make a cut between the anterior and posterior part of this muscle.
- Moving upwards, cut the two heads of the biceps femoris all the way to the hip to expose the sciatic nerve.
- Using blunt forceps and scissors, separate the lateral from the medial head of the gastrocnemius muscle and cut the distal insertion (Achilles tendon) of the medial gastrocnemius muscle. Preserve the fragment of Achilles tendon as long as possible in order to use it to connect to the force transducer.
- Identify the medial gastrocnemius (MG) nerve. Using the forceps and scissors, cut all remaining collaterals of the sciatic nerve, including collaterals to posterior biceps and semitendinosus. Leave the supply blood vessels to the medial gastrocnemius intact.
- Thread a non-elastic ligature through the Achilles tendon and make three knots.
- Place a saline-soaked piece of cotton wool under the exposed nerve and muscle.
- Using serrated forceps, close the skin over the operated area.
- Using sharp-blunt scissors, make a 2 cm incision in the skin and underlying connective tissue along the anterior side of the left hind limb for immobilization with a metal clamp (3.1.6.).
3. Preparation for the recording and stimulation
- Vertebral column and leg fixation and muscle arrangement
- Using a steel clamp, fix the left hind limb by putting the clamp on the tibia.
- Place the rat in the custom-made adjustable frame (isolated copper wire, 1 mm), pull up the skin flaps around the laminectomy using four ligatures and suture the skin to the frame in order to form a pool for paraffin oil (size approximately 50 mm x 50 mm) over the exposed spinal cord.
- Using a Dumont #55 forceps, lift the dura mater at the intersection of the spinal cord, cut it caudally up to the sacral bone and retract it.
- Using a blunt glass rod, separate left and right dorsal and ventral roots at successive levels, taking care not to damage them.
- Fill the pool over the spinal cord with warm (37 °C) paraffin oil, covering the exposed ventral and dorsal roots.
- Place the rat on the custom-made aluminum plate (length 260 mm, width 120 mm, height 80 mm) with a pool (length 135 mm, width 100 mm, depth 45 mm) for his hindlimbs connected to the closed-loop heating system. The plate is a place where the animal will be immobilized and the experiment will be performed.
- Fix the clamp put on the left hindlimb with the metal bar to immobilize the hindimb.
- Fix the vertebral column by putting steel clamps at the sacral bone and the L1 vertebra to immobilize the animal body and eliminate the artefacts in force recordings related to respiratory movements.
- Connect the left medial gastrocnemius muscle with the non-elastic ligature to the force transducer (with compliance of 50 μm/250 mN, measurement range 0-1000 mN) via the Achilles tendon.
- Fill the chamber for hindlimbs with warm (37 °C) paraffin oil to cover the medial gastrocnemius muscle and maintain the oil temperature at 37 °C ± 1 °C using the temperature probe and the automatic system.
- Placement of electrodes for action potentials recording and stimulation
- Insert a bipolar silver-wire electrode through the middle part of the medial gastrocnemius muscle, perpendicular to its long axis. Maintain about 5 mm distance between the two electrodes located along a long axis of the muscle. These electrodes will be used to record motor unit action potentials (MUAPs). Connect the electrodes to the low-noise amplifier.
- Stretch the operated muscle to a passive tension of 100 mN, controlled by the force transducer. For rat medial gastrocnemius at this stretch the MUs of three types develop the highest twitch force15.
- Using sharp-blunt scissors, make a 2 cm incision in the skin of the right hind limb and insert a silver-wire electrode to be used as a reference electrode.
- Place and fix a custom-made insulated metal plate (size 30 mm x 13 mm) above the exposed spinal roots. Put left pairs of ventral and dorsal roots (L4, L5 and L6) on the plate.
- Add saline to the pool formed by the skin around the laminectomy. The saline level should be below the insulated plate.
- Place a silver wire stimulating electrode (two silver wires, 0.5 mm diameter, length 50 mm) over the exposed spinal roots, place a positive pole 3 mm above the plate in oil, whereas the negative pole in the saline (added to the pool, below the plate) and connect to the stimulator.
4. Motor unit recordings
- Stimulating with electrical rectangular pulses (0.1 ms duration, amplitude up to 0.5 V), select the ventral roots (L4, L5 and L6); ventral root stimulation evokes contraction of muscles whereas there is no such effect for dorsal roots. Eliminate dorsal roots from the plate. For the medial gastrocnemius, most axons are in the L5 ventral root.
- Using a pair of Dumont #55 forceps and magnifying glasses, split L5 or L4 ventral roots into very fine bundles of axons (grasp the cut end of the ventral rootlet with both forceps and peel the rootlet apart); place one of these bundles on a silver wire electrode and stimulate (0.1 ms rectangular pulses of amplitude up to 0.5 V) to achieve activity of a single MU. A solid support (metal bar) is very useful for manipulating thin bundles of axons, which can be used as a hand support for using forceps. Note also that an additional source of light is necessary.
- By progressively increasing the intensity of the stimulus, identify a single MU on the basis of the evoked “all-or-none” character of the twitch contraction and action potential stimulus. Carefully test the evoked activity at a stimulation around the threshold.
- When more than one MU is contracting in the studied muscle and increasing level of the force as well as increasing amplitude or changing shape of the action potential are visible, go back to step 4.2 and split the bundle of axons again. Note that the strongest MUs in rat medial gastrocnemius have about 70 times larger twitch force than the weakest ones and when very strong MU is twitching the second, weak MU may be not evident. Note also that some MUs have their muscle fibers located out of the recording area of the electrode and are not visible in electromyogram; in such a case changes in the stimulus amplitude may have effect in the force but not in action potential.
- Stimulate a motor unit with a stimulation protocol necessary for the aim of experiment. For a basic stimulation protocol necessary to calculate all basic motor unit contractile and action potentials properties, include the following.
- Include 5 stimuli at 1 Hz (5 single twitches recorded and averaged; averaging is eliminating noise, which is especially important for the weakest MUs).
- Include a series of stimuli at 10, 20, 30, 40, 50, 60, 75, 100 and 150 Hz frequencies with a duration of 500 ms (these recordings enable calculation of the force-frequency relationship, the maximum tetanic force at 150 Hz as well as the sag at 20-40 Hz stimulation).
- Include the fatigue test (tetani evoked by trains of 14 stimuli at 40 Hz frequency, repeated every second for 4 minutes).
- Include at least 10 s time intervals between all the elements of the protocol above.
- Repeat the process with successive isolated motor units.
- Terminate the experiment and euthanize the animal using intraperitoneal administration of a lethal dose of pentobarbital sodium (180 mg·kg-1).
5. Electronic apparatus
NOTE: The custom-made computer program controls the stimulator, providing the possibility to create variable patterns of stimulation, including those indicated in step 4.4. The program cooperates with the analog-to-digital converter (at least 10 kHz for the MUAP and force recordings).
- Connect the AC amplifier to the computer by the analog-to-digital converter and in parallel to the oscilloscope.
- Connect the force transducer to the computer by the analog-to-digital converter and in parallel to the oscilloscope. Use the force transducer to control the passive muscle force during experiment. Note that during experiment, the passive force may decrease; therefore, it is necessary to increase the muscle length to keep the passive muscle force constant.
Representative Results
Parameters of motor unit contractions and action potentials can be calculated on the basis of recordings when stable conditions of recordings are ensured. Figure 1 presents a representative recording of the single twitch of a fast MU. The upper trace shows the motor unit action potential. The delay between stimulus delivery and onset of the motor unit action potential is due to conduction time from ventral root to muscle. Figure 2 shows a representative recording of the unfused tetanus force of a fast MU and a train of motor unit action potentials.
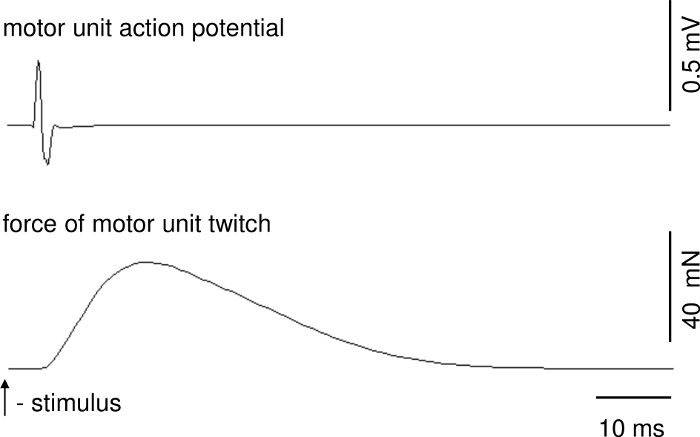
Figure 1: A representative recording of the single twitch of a fast MU. Over the force track, there is motor unit action potential. Please click here to view a larger version of this figure.
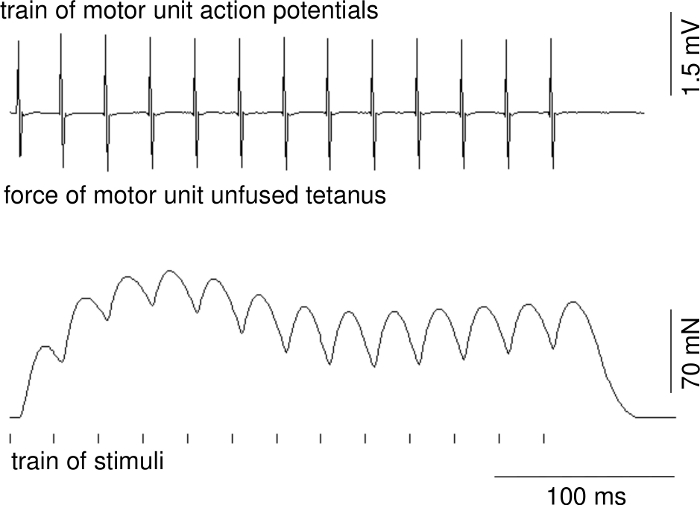
Figure 2: A representative recording of the unfused tetanus force of a fast MU (middle recording), a train of motor unit action potentials (upper recording) and a time position of a train of applied stimuli (below). Please click here to view a larger version of this figure.
Discussion
If performed correctly by experienced scientists, the surgical component of the described protocol should be completed within approximately two hours. One should take particular care to maintain stable physiological conditions of the animal during the surgery, particularly body temperature and depth of anesthesia, which should be systematically controlled by assessing pinna and withdrawal reflexes. Following the surgery, it should be possible to maintain stable recording conditions for at least six hours.
The crucial experimental procedure begins with the splitting of the ventral root into very thin filaments leading to isolation of a single motor axon to the studied muscle. In fact, the thin filaments of ventral roots contain groups of axons innervating different muscles of the hindlimb; however, because all muscles except the studied one are denervated, when the stimulated bundle of axons contains only one axon to the studied medial gastrocnemius it is possible to evoke the single MU contraction only in this studied muscle. Following successful identification of the evoked activity as single MU contraction, it is possible to record a set of force recordings (single twitch, the unfused tetanus, the fatigue test) crucial for a classification of MU as one of three physiological types. The advantage of this technique is the ability to record up to 30 units in one experiment; additionally, MUs can be immediately classified as fast or slow types on the basis of “sag” presence1,3. Furthermore, MUs can be classified as fast-fatigable or fast-resistant with very high accuracy based on a profile of the unfused tetanic contraction force recording16. This last method may be used when the classical fatigue test cannot be performed. It is also worth noting that fast/slow MU classification can be also done with a 20 Hz index17.
The proposed stimulation protocol (step 4.4) may be adapted to needs of the study. This particular set of stimulations enable to record twitch (to calculate basic twitch parameters including the twitch force, contraction as well as relaxation time), the maximum tetanus (therefore it is possible to calculate the twitch-to-tetanus ratio), unfused tetanic contractions at a set of stimulation frequencies (to classify an MU as slow or fast basing on sag presence or 20 Hz index, as well as to calculate the force-frequency curve) and the fatigue test (necessary to calculate the fatigue index). The fatigue index calculation is a basic method to classify MUs as fatigable or resistant. This method is open to being modified to produce various stimulation patterns; however, a possible limitation is the computer program that generates the time distribution of stimuli delivered to the axon. Moreover, some additional modifications may be introduced to answer specific research questions, such as several stimulating electrodes to activate several MUs in parallel18, an additional laser sensor to record a mechanomyogram (MMG) from the muscle surface19 or a recording electrode on a nerve branch to the muscle to calculate nerve conduction velocity20.
However, it is important to be aware of the limitations and challenges of this procedure. First, a considerable part of the experimental setup is custom-made (i.e., clamps for the limb and the vertebrae segments, a plate for ventral roots and electrodes). The experimental setup includes a solid metal table with plate (thickness 30 mm) for all supporting metal bars (necessary for animal immobilization and the force transducer) to enable stable conditions for the isometric force recording. The application of this method also requires both extensive training in surgery as well as preparation of a complex experimental setup including an electronic apparatus and a computer program.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by Polish National Research Centre grant 2018/31/B/NZ7/01028.
Materials
| Force transducer | custom-made | ||
| Forceps | Fine Science Tools | No. 11255-20 | Dumont #55 with extra light and fine shanks |
| Forceps | Fine Science Tools | No. 11150-10 | Extra Fine Greafe Forceps |
| Forceps | Fine Science Tools | No. 11026-15 | Special cupped pattern for superior grip |
| Forceps | Fine Science Tools | No. 11023-10 | Slim 1×2 teeth |
| Forceps | Fine Science Tools | No. 11251-20 | Dumont #5 |
| Hemostats | Fine Science Tools | No. 13003-10 | Hartman |
| Isolation Unit | Grass Instruments | S1U5A | |
| Low Noise Bioamplifer | World Precision Instruments | Order code 74030 | |
| Needle holders | Fine Science Tools | No. 12503-15 | With tungsten carbide jaws |
| Rongeurs | Fine Science Tools | No. 16021-14 | Friedman-Pearson |
| Scissors | Fine Science Tools | No. 14101-14 | Straight sharp/blunt with large finger loops |
| Scissors | Fine Science Tools | No. 14075-11 | Curved blunt/blunt |
| Scissors | Fine Science Tools | No. 14084-08 | Extra fine bonn |
| Scissors | Fine Science Tools | No. 15000-00 | Straight, ideal for cutting nerves |
| Stimulator | Grass Instruments | S88 | Dual Output Square Pulse Stimulator |
References
- Burke, R. E., Levine, D. N., Tsairis, P., Zajac, F. E. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. Journal of Physiology. 234, 723-748 (1973).
- Celichowski, J., Drzymała-Celichowska, H. The number of motor units in the medial gastrocnemius muscle of male and female rats. Journal of Physiology and Pharmacology. 58, 821-828 (2007).
- Grottel, K., Celichowski, J. Division of motor units in medial gastrocnemius muscle of the rat in light of variability of their principal properties. Acta Neurobiologiae Experimentalis. 50, 571-588 (1990).
- Celichowski, J., Krutki, P. Variability and plasticity of motor unit properties in mammalian skeletal muscle. Biocybernetics and Biomedical Engineering. 32 (4), 33-45 (2012).
- Gardiner, P. F., Olha, A. E. Contractile and electromyographic characteristics of rat plantaris motor unit types during fatigue in situ. Journal of Physiology. 385, 13-34 (1987).
- Drzymała-Celichowska, H., Kaczmarek, P., Krutki, P., Celichowski, J. Summation of slow motor unit forces at constant and variable interpulse intervals in rat soleus muscle. Journal of Electromyography and Kinesiology. 30, 1-8 (2016).
- Krutki, P., Celichowski, J., Łochyński, D., Pogrzebna, M., Mrówczyński, W. Interspecies differences of motor units properties in the medial gastrocnemius muscle of cat and rat. Archives Italiennes de Biologie. 144, 11-23 (2006).
- Burke, R. E., Rudomin, P., Zajac, F. E. The effect of activation history on tension production by individual muscle units. Brain Research. 109, 515-529 (1976).
- Celichowski, J. Mechanisms underlying the regulation of motor unit contraction in the skeletal muscle. Journal of Physiology and Pharmacology. 51, 17-33 (2000).
- Burke, R. E., Levine, D. N., Salcman, M., Tsairis, P. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. Journal of Physiology. 238, 503-514 (1974).
- Milner-Brown, H. S., Stein, R. B., Yemm, R. The contractile properties of human motor units during voluntary isometric contractions. Journal of Physiology. 228, 285-306 (1973).
- Taylor, A., Stephens, J. A. Study of human motor unit contractions by controlled intramuscular microstimulation. Brain Research. 117, 331-335 (1976).
- Westling, G., Johansson, R. S., Thomas, C. K., Bigland-Ritchie, B. Measurement of contractile and electrical properties of single human thenar motor units in response to intraneural motor-axon stimulation. Journal of Neurophysiology. 64, 1331-1338 (1990).
- Burke, R. E. Motor units: anatomy, physiology and functional organization. APS Handbook of Physiology Series, Section 1, The Nervous System. 11, 345-422 (1981).
- Celichowski, J., Grottel, K. The dependence of the twitch course of medial gastrocnemius muscle of the rat and its motor units on stretching of the muscle. Archives Italiennes de Biologie. 130, 315-325 (1992).
- Celichowski, J., Grottel, K., Bichler, E. Differences in the profile of unfused tetani of fast motor units with respect to their resistance to fatigue in the rat medial gastrocnemius muscle. Journal of Muscle Research and Cell Motility. 20, 681-685 (1999).
- Krutki, P., et al. Division of motor units into fast and slow on the basis of profile of 20 Hz unfused tetanus. Journal of Physiology and Pharmacology. 59, 353-363 (2008).
- Drzymała-Celichowska, H., Krutki, P., Celichowski, J. Summation of motor unit forces in the rat medial gastrocnemius muscle. Journal of Electromyography and Kinesiology. 20, 599-607 (2010).
- Kaczmarek, P., Celichowski, J., Drzymała-Celichowska, H., Kasiński, A. The image of motor unit architecture in the mechanomyographic signal during single motor unit contraction. In vivo and simulation study. Journal of Electromyography and Kinesiology. 19, 553-563 (2009).
- Celichowski, J., Krutki, P., Bichler, E. Axonal conduction velocity of motor units of rat’s medial gastrocnemius muscle. Journal of Physiology (Paris). 90, 75-78 (1996).

