Biotin-based Pulldown Assay to Validate mRNA Targets of Cellular miRNAs
Summary
This report describes a fast and reliable method for validating mRNA targets of cellular miRNAs. The method uses synthetic biotinylated Locked Nucleic Acid (LNA)-based miRNA mimics to capture target mRNA. Subsequently, streptavidin-coated magnetic beads are employed to pulldown the target mRNA for quantification by qPCR polymerase chain reaction.
Abstract
MicroRNAs (miRNAs) are a class of small noncoding RNAs that post-transcriptionally regulate cellular gene expression. MiRNAs bind to the 3' untranslated region (UTR) of target mRNA to inhibit protein translation or in some instances cause mRNA degradation. The binding of the miRNA to the 3' UTR of the target mRNA is mediated by a 2–8 nucleotide seed sequence at the 5' end of miRNA. While the role of miRNAs as cellular regulatory molecules is well established, identification of the target mRNAs with functional relevance remains a challenge. Bioinformatic tools have been employed to predict sequences within the 3' UTR of mRNAs as potential targets for miRNA binding. These tools have also been utilized to determine the evolutionary conservation of such sequences among related species in an attempt to predict functional role. However, these computational methods often generate false positive results and are limited to predicting canonical interaction between miRNA and mRNA. Therefore, experimental procedures that measure direct binding of miRNA to its mRNA target are necessary to establish functional interaction. In this report, we describe a sensitive method for validating direct interaction between the cellular miRNA miR-125b and the 3' UTR of PARP-1 mRNA. We elaborate a protocol in which synthetic biotinylated-miRNA mimics were transfected into mammalian cells and the miRNA-mRNA complex in the cellular lysate was pulled down with streptavidin-coated magnetic beads. Finally, the target mRNA in the pulled-down nucleic acid complex was quantified using a qPCR-based strategy.
Introduction
MicroRNAs (miRNAs) are small non-coding RNAs that negatively regulate protein expression1. Precursors of miRNA reside in clusters through many regions of the genome, most frequently within the intergenic regions and introns of protein-coding genes2,3. Biogenesis of miRNAs involve transcription of the pri-miRNAs from miRNA-encoding genes4. The pri-miRNAs undergo sequential processing, first in the nucleus and then in the cytoplasm to generate single stranded mature miRNAs4,5. Subsequently, the mature miRNAs are incorporated into the RNA-induced silencing complex (RISC): a multimeric protein-RNA complex that includes a member of the Argonaute family of proteins for target recognition4,5,6,7. Mature miRNAs in the RISC complex predominantly bind to the 3' UTR of target mRNAs8,9,10 but can also bind occasionally in the coding and the 5'UTR region of the mRNA10,11. The binding of miRNA to the mRNA sequences results in translational silencing12,13,14 and in some cases mRNA destabilization15. Since a single miRNA can target many mRNAs, these regulatory molecules are involved in almost every cellular process and have been implicated in various disease conditions16,17,18.
Detailed understanding of how miRNAs regulate cellular pathways requires identification of the target mRNAs. Multiple bioinformatics platforms are available to predict putative miRNA:mRNA interactions19,20. These predictions rely on perfect Watson-Crick base pairing between the 2–8 nucleotide seed sequence of the miRNA and a complementary sequence within the target mRNA4,21. Additionally, these tools generate secondary structure of the miRNA:mRNA duplex, calculate thermodynamic parameters of this molecular interaction and show conservation of the binding sites across species to enhance functional relevance of the target prediction. Unfortunately, these tools also have the limitation of predicting false positive targets at a very high rate (~27–70%)15,22. Most importantly, these in silico platforms fail to recognize the non-canonical interactions of miRNAs with their targets23. Therefore, such predictive analyses are often combined with experimental methods to validate functionally relevant targets.
Multiple approaches have been developed to experimentally validate miRNA:mRNA interaction. Genetic experiments using miRNA mimics, sponges and inhibitors that alter the levels of miRNAs in the cell provide clues for its regulatory effect on the target gene expression24,25,26. Additionally, reporter based assays via co-transfection of a clone containing the 3' UTR region of the target mRNA and miRNA mimics or inhibitors into cells provide evidence of the regulatory function of miRNAs26. While these methods are crucial to study post-transcriptional regulation of gene expression by miRNAs, transfection efficiency and pleotropic effects of alterations in cellular miRNA levels are major limitations of these genetic approaches23,26. Therefore, complementary biochemical methods that probe direct interaction between miRNA and its target are employed to better understand the cellular function of miRNAs.
One widely-used method to study direct interaction between miRNA and its target is immunoprecipitation of the RISC complex followed by the detection of mRNA target within the complex27,28,29,30. Recently, an improved RISC trap method was also utilized to identify miRNA targets; it couples stabilization of targets within the RISC-miRNA-mRNA intermediates with the purification of mRNA targets. However, these methodsface the inherent challenges of non-specific interactions between RNA and RNA-binding proteins that are usually segregated by cellular compartments31,32. In addition, these assays are dependent on the presence of AGO2 protein for immunoprecipitation of RISC complex33. Given that AGO2 is not the only argonaute to mediate efficient miRNA:mRNA interactions, exclusion of other argonautes could lead to biased results34. Therefore, alternative strategies are needed to study direct binding of miRNA to mRNA.
In this report, we elaborate a one-step approach for probing direct interaction between a miRNA and its target mRNA. First, 3' biotinylated locked nucleic acid (LNA) miRNA mimics are transfected into mammalian cells. Then, the miRNA:mRNA complex in the cellular lysate is captured using streptavidin coated magnetic beads. The mRNA target bound to its complementary miRNA is quantified using qPCR.
Protocol
1. Transfection of 3'-biotinylated miRNA
NOTE: Perform the following steps inside a sterile laminar flow hood.
- Seed 4 x 105–5 x 105 HEK-293T cells per well in 2 mL of complete Dulbecco's Modified Eagle Medium (DMEM) [DMEM supplemented with 10% Fetal Bovine serum (FBS) and 1x Pen-strep antibiotic]. Culture the cells in an incubator set at 37 °C, 5% CO2 overnight.
- The next day, check the health and adherence of the plated cells under a light microscope.
NOTE: Make sure the cells have adhered and attained appropriate morphology before proceeding to the next step. The HEK-293T cells are typically ready for transfection 18–24 h post plating. - In a sterile 1.7 mL microfuge tube, dilute 75 picomoles of biotinylated miRNA in 200 µL of minimal essential media. Transfer this mix to another 1.7 mL microfuge tube containing Liposome based transfection reagent (8 µL/well) diluted in 200 µL of minimal essential media. Mix thoroughly, but gently, by pipetting and incubate for 20–25 min at room temperature to allow formation of the transfection complexes.
- Remove the old media from the 6 well culture plate by gentle pipetting and replenish each well with 1.6 mL of freshly prepared complete DMEM (without 1x Pen-strep antibiotics).
- Add the transfection complexes (from 1.3) drop-wise to the cells in the 6-well plate using a well-calibrated pipette. Swirl the plate gently while adding the complexes to ensure their uniform distribution across the plate.
NOTE: This step is very critical for achieving high-efficiency transfection and must be performed with care and patience. - Culture the cells at 37 °C and 5% CO2 for at least 36 h.
2. Preparation of Streptavidin Coated Magnetic Beads — I
- Resuspend the streptavidin magnetic beads thoroughly by vortexing. Transfer 30 µL of bead suspension (per sample) to a 2 mL nuclease-free microfuge tube.
- Place the tube containing the bead suspension on the magnetic bead separator stand ("magnet" hereafter) for 2 min. After ensuring that the beads are drawn to the side of the tube in contact with the magnet, carefully remove the supernatant using a micropipette.
- Add 100 µL of bead wash buffer (10 mM Tris-Cl pH 7.5, 0.5 mM EDTA, 1 M NaCl) to the beads. Remove the tube from the magnet. Vortex for 15 s at room temperature to wash the beads.
- Place the tube containing beads on the magnet for 2 min, and carefully remove and discard the supernatant.
- Repeat steps 2.3 and 2.4 for a total of three washes. After the final wash step, remove the tube from the magnet.
- Add 100 µL of RNase freeing solution (0.1 M NaOH, 0.05 M NaCl) to the beads, mix well by vortexing for 15 s, and incubate at room temperature for 5 min. Place the tube containing beads on the magnet for 2 min, and carefully remove and discard the supernatant.
- Repeat step 2.6 for a total of three times.
- Add bead resuspension solution (0.5 M NaCl) to the beads, vortex 15 s, and incubate at room temperature for 5 min. Place the resuspended beads on the magnet for 2 min, and carefully remove the supernatant.
- Add 200 µL of bead blocking solution (1 µg/µL BSA, 2 µg/µL Yeast tRNA) to the beads. Mix by gentle vortexing for 15 s at room temperature. Incubate at 4 °C for 16 h (over-night) on a multi-tube rotator.
3. Preparation of Cell Lysates
- Harvest the transfected HEK-293T cells by gentle scraping using a sterile cell scraper inside the laminar hood, then transfer each sample into a sterile 2 mL microfuge tube.
- Pellet the scraped cells by centrifugation at 1,500 x g for 5 min. Resuspend the pellet in 1x sterile PBS (Phosphate Buffer Saline), pH 7.2. Centrifuge again at 1,500 x g for 5 min to obtain a pellet free of residual media. Immediately plunge the tube containing pellet into ice.
- Prepare fresh complete cell lysis buffer (150 mM NaCl, 25 mM Tris-Cl, pH-7.5, 5mM DTT, 0.5% IGEPAL, 60 U/mL Superase, 1x Protease Inhibitor).
NOTE: A stock of cell lysis buffer lacking IGEPAL, Superase, and Protease inhibitor cocktail may be prepared in advance and stored at room temperature. Prepare a fresh batch of complete cell lysis buffer by adding the above supplements at the recommended final concentrations to the stock cell lysis buffer and storing it on ice. - Add 260 µL of ice-cold complete cell lysis buffer to each sample in the microfuge tube (i.e., each microfuge tube contains cells harvested from one well of the 6-well plate) and resuspend the cell pellet into a homogenous suspension by pipetting.
- Lyse the cells using the freeze-thaw method: Incubate the tubes at -80 °C for 10–15 min. Then, allow the cells to thaw out on ice.
- Centrifuge the resulting cell lysate at 16,000 x g for 5 min in a refrigerated benchtop centrifuge set at 4 °C.
- Transfer the cleared cell lysate to a sterile 1.7 mL microfuge tube on ice. Discard the pellet.
NOTE: The final volume of cleared lysate should be ~240–250 µL. - Add 5M NaCl to the cleared lysate to a final concentration of 1M and maintain the samples on ice.
4. Preparation of Streptavidin Magnetic Beads — II
- Prepare fresh complete pull-down wash buffer (10 mM KCl, 1.5 mM MgCl2, 10 mM Tris-Cl pH 7.5, 5 mM DTT, 1 M NaCl, 0.5% IGEPAL, 60U/mL Superase, and 1x Protease Inhibitor cocktail)
NOTE: A stock of pull-down wash buffer lacking IGEPAL, Superase, and Protease inhibitor cocktail may be prepared in advance and stored at room temperature. Prepare a fresh batch of complete cell lysis buffer by adding the above supplements at the recommended final concentrations to the stock cell lysis buffer and storing it on ice. - Place the tube containing the prepared beads (from step 2.9) on the magnet for 2 min. Carefully remove and discard the supernatant.
- Add 150 µL of ice-cold complete pull-down wash buffer to the beads. Vortex for 15 s and incubate at room temperature for 30–60 s. Place the tubes on the magnet for 2 min. Carefully remove and discard the supernatant.
- Repeat step 4.3 for a total of 3 times.
- Resuspend the beads in 300 µL of complete pull-down wash buffer.
5. Pull-down of Target mRNA-miRNA Complexes
- Transfer 300 µL of the cell lysate (from step 3.8) to the microfuge tube containing 300 µL of beads (from step 4.5).
- Incubate the mixture on a nutating mixer for 1 h at room temperature.
- Place the tube on the magnet for 5 min. Carefully remove and discard the supernatant.
- Add 300 µL of ice-cold complete pull-down wash buffer to the beads. Vortex for 15 s at room temperature and place the tube on the magnet for 5 min. Carefully remove and discard the supernatant.
- Repeat step 5.4 two more times.
- Resuspend the beads in 100 µL of nuclease-free water and incubate on ice.
6. Total RNA Extraction, cDNA Synthesis, and qPCR
- Extract total RNA from the resuspended beads (from step 5.6) using an appropriate method (see Table of Materials). Resuspend the extracted total RNA in 25 µL of nuclease-free water. Determine the RNA concentration and quality using a spectrophotometer (absorbance at 260/280). Prepare a working stock of RNA at 50 ng/µL.
- Perform cDNA synthesis, in triplicates, using 50 ng of total RNA (from step 6.1) using an appropriate cDNA synthesis kit (see Table of Materials) using oligo dT primers in a final volume of 20 µL (Table 1). Then, load PCR tubes into the qPCR instrument to perform thermal cycling as per the conditions described in Table 2.
- Perform qPCR on the CDNA (from step 6.2) using SYBR Green chemistry (see Table of Materials):
- Perform all qPCR reactions in triplicates in a 96 well clear bottom plate in sterile conditions.
- Aliquot 9 µL of the reaction mixture from the qPCR master mix (see Table 3) into each well of the plate. Then, add 1 µL of the cDNA to each well to achieve a final volume of 10 µL. Seal the PCR plate using heat resistant PCR plate sealer and load into the qPCR instrument
- Perform thermal cycling as per the conditions described in Table 4.
- Normalize the expression levels (Ct values) of each sample to the expression levels of the scrambled control. Use these values to calculate fold changes in expression.
Representative Results
MiRNAs regulate cellular processes by binding to target mRNAs. Therefore, identifying mRNA targets are a key to understand miRNA function. Here we elaborate a method for identifying mRNA target of cellular miRNA. This protocol is adapted from Wani et al.35 with the modification of utilizing biotinylated LNA-based miRNA mimics to pulldown target mRNA. The use of LNA-based oligonucleotide mimics enhances specificity of target binding owing to the higher stability of the modified ribose ring without toxic effects36,37,38. We employed this method to pull down PARP-1 mRNA as the target of cellular miRNA miR-125b. The highest level of miR-125b expression is detected in brain tissue that regulates neuronal function39,40. In an attempt to identify the targets of miR-125b in neuronal cells, recently we reported that miR-125b negatively regulates PARP-1 expression by binding to the 3' UTR of PARP-1 mRNA41.
Pulldown of miRNA:mRNA Complex
We used HEK-293T cells to study the interaction between miR-125b and PARP-1 mRNA, since these cells are widely used in cellular, biochemical, and molecular biology studies. A schematic of the protocol used for the mRNA target is depicted in Figure 1. First, HEK-293T cells (4 x 105–5 x 105 cells per well) were seeded overnight in a 6-well tissue culture plate and incubated at 37 °C with 5% CO2 for 18–24 h. Thereafter, 75 picomoles of 3'-biotinylated miR-125b mimics and 3'-biotinylated scrambled controls were transfected into the cells using liposome based transfection method. Transfected cells were incubated at 37 °C and 5% CO2 for an additional 36 h and harvested. Thereafter cellular lysates of transfected cells were prepared by the freeze-thaw lysis method. The freshly prepared cellular lysates were incubated with the blocked streptavidin coated magnetic beads on a bench top nutating mixer for 1 h at room temperature. Following this, the beads were processed and washed using freshly prepared ice cold pull-down wash buffer on the magnetic separator. Finally, the beads were resuspended in 100 µL of nuclease free water for further analysis.
Detection of Target mRNA in the pull-down miRNA:mRNA Complex
To detect the target mRNA of miR-125b from the pull-down mixture, we employed a qPCR assay (Figure 1). We designed forward and reverse primers (FP and RP) within the ORF to amplify PARP-1 mRNA (Figure 2, Table 5b). We also designed primers sets for detecting p53 mRNA, a known target of miR-125b42, as a positive control and included primers to amplify actin mRNA as a non-specific negative control. In addition, to further confirm the specificity of amplification, we designed primers for detecting the 3' UTR regions of PARP-1, p53 and actin. We performed qPCR using the methods described in the protocol (section 6).
Figure 3 shows the qPCR based amplification of target mRNA's relative to scrambled controls from the purified beads. The level of PARP-1 mRNA was markedly higher in samples pulled-down with biotinylated miR-125b mimics when compared to the scrambled controls. As expected, higher mRNA levels of the positive control p53 mRNA and minimal amplification of the negative control actin mRNA was observed in these samples. Similar levels of amplification were obtained using the primers targeting the 3' UTR of PARP-1 and p53 compared to the negative control actin (Figure 3B). The qPCR results of the ORF regions (Figure 3A) and the 3'UTR regions (Figure 3B) of PARP-1 mRNA and p53 mRNA showed some levels of variability. Several factors including binding affinity, number of miRNA binding sites, and differences in primer sequence dependent amplification may contribute to the variable levels of amplification in Figure 3. Nevertheless, these data strongly support the feasibility and specificity of the method for validating mRNA targets of cellular miRNAs.
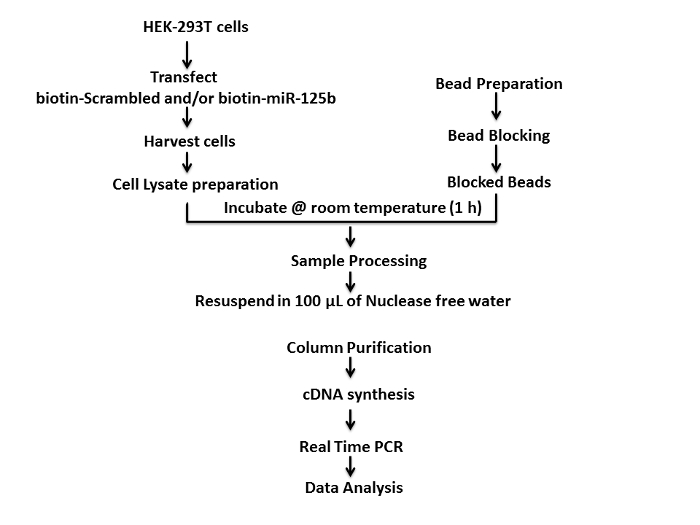
Figure 1: Schematic representation of the protocol for the target capture assay using biotinylated-miRNA mimics. Please click here to view a larger version of this figure.
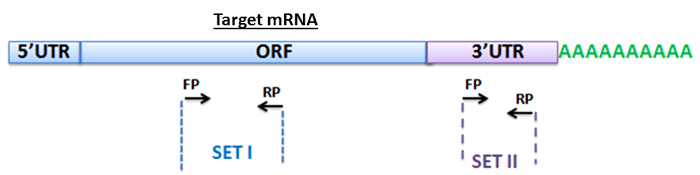
Figure 2: Primer design for amplification of mRNA targets. Schematic representation of the primers used to detect the target mRNA of miR-125b. One set of primers (Set I) was designed within the ORF region of the transcripts and the other set of primers (Set II) was designed in the 3' UTR region of the target genes. FP and RP represent forward and reverse primers for each set. Please click here to view a larger version of this figure.
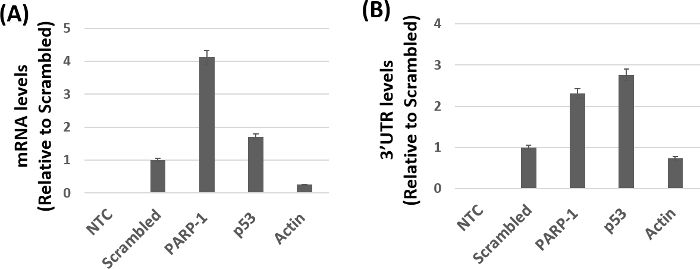
Figure 3: miR-125b target identification by qPCR. (A) mRNA levels using primer set I, which amplifies the ORF region of target mRNA. Higher expression levels of PARP-1 and p53 mRNA (positive control) were observed when compared to actin mRNA (negative control). (B) Amplification of the 3' UTR region of target mRNA using primer set II. No amplification was observed in the no template controls (NTC). All reactions were performed in triplicates and the data was analyzed using the appropriate data analysis software based on the 2-ΔCt method. Error bars represent standard error of the mean. Please click here to view a larger version of this figure.
| Component | Stock | Final volume for 20 μL reaction |
| Template (RNA) | 50 ng/µL | 1.0 μL |
| Reaction buffer | 5x | 4.0 μL |
| Oligo dT primer | Master stock | 2 μL |
| Reverse Transcriptase | Master stock | 1 μL |
| Nuclease-free distilled water | – | 12 μL |
| Total Reaction Volume | 20 μL |
Table 1: cDNA synthesis reaction mixture.
| 105 °C = 30 s |
| 42 °C = 60 min |
| 95 °C = 5 min |
| 10 °C = Hold |
Table 2: cDNA synthesis thermal cycling conditions.
| Component | Stock | Final volume for 10 μL reaction |
| cDNA product | – | 1.0 μL |
| iTaq Universal SYBR mix | 2x | 5.0 μL |
| Target (ORF/3’ UTR) F.P. | 10 μM | 0.5 μL |
| Target (ORF/3’ UTR) R.P. | 10 μM | 0.5 μL |
| Nuclease-free distilled water | – | 3 μL |
| Total Reaction Volume | 10 μL |
Table 3: qPCR reaction mixture.
| 95 °C = 10 min |
| 30 cycles of: |
| 95 °C = 10 s |
| 56 °C = 30 s |
| 72 °C = 30 s |
| Melt Curve Analysis |
| 65 °C= 31 s |
| Linear Ramp rate = 0.5 °C/s |
| Acquisition = 0.5 °C intervals |
Table 4: qPCR thermal cycling conditions.
| (A): Biotinylated Oligos | ||
| Biotinlyated Oligos | Stock | Sequence (5' to 3’) |
| hsa-miR-125b | 25 μM | UCCCUGAGACCCUAACUUGUGA-Biotin |
| Scrambled Control | 25 μM | GAUGGCAUUCGAUCAGUUCUA-Biotin |
| (B) Primers | ||
| Target Primer (ORF) | Stock | Sequence (5’ to 3’) |
| PARP-1 (FP) | 100 μM | GAGGTGGATGGGTTCTCTGA |
| PARP-1 (RP) | 100 μM | ACACCCCTTGCACGTACTTC |
| p53 (FP) | 100 μM | TGTGACTTGCACGTACTCCC |
| p53 (RP) | 100 μM | ACCATCGCTATCTGAGCAGC |
| ACTIN (FP) | 100 μM | GCTCGTCGTCGACAACGGCTC |
| ACTIN (RP) | 100 μM | CAAACATGATCTGGGTCATCTT |
| (C) Primers | ||
| Target Primer (3’ UTR) | Stock | Sequence (5’ to 3’) |
| PARP-1 (FP) | 100 μM | ATTGGGAGAGGTAGCCGAGT |
| PARP-1 (RP) | 100 μM | CTACCCATCAGCAACTTAGCG |
| p53 (FP) | 100 μM | GGCCCATATCTGTGAAATGC |
| p53 (RP) | 100 μM | CTGCAGGAAGGCAGGTTTT |
Table 5: Oligonucleotide Names and Sequences.
Discussion
Identification of mRNA targets of cellular miRNAs is important for understanding their regulatory function. Several computational tools are employed to predict targets based on seed sequence complementarity and conservation of target sequences19,20. Although these tools are valuable, they can generate high levels of both false positives and false negatives. Therefore, these predictions are coupled with experimental methods such as immunoprecipitation of RISC complex and pull-down of miRNA:mRNA complex to confirm direct binding of miRNAs to their respective targets27,31. This report details an experimental strategy that utilizes biotinylated miRNA mimics to pulldown target mRNA. Biotin pulldown can be used for identification of miRNA targets with high sensitivity and low false positive rate35,43. Therefore, this method can also be exploited for identification of miRNA targets by coupling sequence-based screening of the captured RNA pool. However, there are several limitations to this method44. Over-expression of an exogenous miRNA could modify the transcriptional network of cells, causing consequent changes in mRNA that are not due to miRNA regulation. These could be overcome by using very low amounts of mimics so as not to perturb endogenous miRNA regulation. Also, proper controls are required when miRNAs are exogenously expressed to avoid non-specific binding. Careful washing and blocking steps can effectively minimize background noise and increase specificity of the targeted miRNA. Few studies have shown that modifications of miRNA 3' or 5' ends are not well tolerated45, nevertheless, several studies have efficiently used biotin tagged miR-mimics as an effective tool to explore mRNA targets.
The other advantage of this method is the utilization of LNA-based (locked nucleic acid) miRNA mimics. The LNA nucleic acid modification allows formation of stable oligonucleotides duplexes with high affinity46,47. Moreover, the custom made biotinylated LNA miRNA mimics consist of three RNA strands. A 3'-biotinylated miRNA strand with sequence as per the miRBase annotation and a passenger strand complementary to the miRNA that is split in two LNA-modified RNA strands47. RISC incorporates only the miRNA strand while the two passenger strands are rapidly degraded thus enhancing target specificity. To increase the confidence of our findings, we have also included a validated target p53 of miR-125b as a positive control and β-actin, which does not have a miR-125b binding site in its mRNA as a negative control. The results obtained using this method with these controls (Figure 3) show specificity of target mRNA identification. Although LNA miRNA mimics stabilize and greatly favor Watson-Crick base pairing, it should be pointed out that the use of LNA miRNA mimics with biotin label will eliminate neither false positives nor negatives. Moreover, it is likely that they may result in confirmation of computational predictions that may not be biologically relevant. On the other hand, if this method is coupled with sequencing to identify mRNA targets, false negatives may be generated, because LNA mimics favor canonical base pairing that may exclude the non-canonical base pairing interactions that occur with native miRNAs.
The protocol presented here is adapted from Wani et al, with several modifications35. To further reduce background noise, we have incorporated extensive washing steps, modified the parameters for transfection efficiency, buffer preparation and incubation, pulldown conditions and PCR data acquisition and analysis. In addition, we used two primer sets to amplify the target mRNA after the pull-down, one set designed to amplify a region of the ORF of target mRNA and the second set of primers to amplify a region within the 3' UTR of the target mRNA. Utilization of two sets of primers enhances the specificity for the target mRNA enrichment.
It should be noted that a limitation of this method is a basal level of noise that arises from non-specific targets. Further modifications such as improvements in the blocking, washing and incubation conditions may provide higher target specificity and sensitivity. In summary, the assay described in this report is a fast and reliable method that can be adopted to validate mRNA targets of miRNAs using a PCR based strategy.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was partly supported by National Institutes of Health (NIH) Grants DA037779 (to J.P.), DA024558, DA30896, DA033892, DA021471, AI22960 and MD007586 (to C.D.). The work was also supported by the RCMI Grant G12MD007586, the Vanderbilt CTSA Grant UL1RR024975, the Meharry Translational Research Center (MeTRC) CTSA grant (U54 RR026140 from NCRR/NIH, the U54 Grant MD007593 from NIMHD/NIH, and the Tennessee Center for AIDS Research (P30 AI110527).
Materials
| Cell line- HEK293T cells | ATCC | CRL-3216 | |
| Heat-inactivated Fetal bovine serum (Hi-FBS) | GIBCO/Thermofisher | 10438-026 | |
| Dulbecco’s modified Eagle’s medium (DMEM) | GIBCO/Thermofisher | 11995-065 | |
| Phosphate buffered saline (PBS) (1x) | GIBCO/Thermofisher | 20012-027 | |
| Trypsin-EDTA (0.25%) | GIBCO/Thermofisher | 25200-056 | |
| Penicillin-Streptomycin solution (100x) | Cellgro/Mediatech | 30-002-CI | |
| DNase | Ambion | AM2238 | |
| Opti-MEM Reduced serum media | GIBCO/Thermofisher | 3198-088 | |
| Liopfectamine 2000 Transfection reagent | Invitrogen/ Thermofisher | 11668-019 | |
| Biotinylated hsa-miR-125b | Exiqon | 339178/ID-27281622 | |
| Biotinylated scrambled control | Exiqon | 479997-671/ID-714884 | |
| IGEPAL | Sigma-Aldrich | 18896 | |
| Streptavidin Magnetic beads | Pierce | 88816 | |
| Yeast tRNA | Invitrogen/Thermofisher | 15401-011 | |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | A2153 | |
| Potassium chloride (KCl) solution | Sigma-Aldrich | 60142 | |
| Magnesium chloride (MgCl2) solution | Sigma-Aldrich | M1028 | |
| Sodium hydroxide (NaOH) | Sigma-Aldrich | 795429 | |
| Ethylenediamine tetraacetic acid, disodium salt (EDTA) solution, 0.5 M, pH 8.0 | Sigma-Aldrich | E7889 | |
| Dithiothreitol (DTT) | Sigma-Aldrich | 43815 | |
| Superase | Ambion/Thermofisher | AM2694 | |
| Protease Inhibitor cocktail | Sigma-Aldrich | P8340 | |
| RNAse/DNAse free water | GIBCO/Thermofisher | 10977-015 | |
| RNeasy extraction kit | Qiagen | 74104 | |
| iScript-Select cDNA synthesis kit | Biorad | 170-8897 | |
| qPCR Primers | Invitrogen/Thermofisher | ||
| iTaq-Universal SYBR green supermix | Biorad | 172-5120 | |
| DynaMag-Spin Magnet | Invitrogen/Thermofisher | 12320D |
References
- Behm-Ansmant, I., Rehwinkel, J., Izaurralde, E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harborsymposia on quantitative biology. 71, 523-530 (2006).
- Rodriguez, A., Griffiths-Jones, S., Ashurst, J. L., Bradley, A. Identification of mammalian microRNA host genes and transcription units. Genome research. 14, 1902-1910 (2004).
- Lagos-Quintana, M., Rauhut, R., Meyer, J., Borkhardt, A., Tuschl, T. New microRNAs from mouse and human. RNA. 9, 175-179 (2003).
- Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116, 281-297 (2004).
- Lee, Y., Jeon, K., Lee, J. T., Kim, S., Kim, V. N. MicroRNA maturation: Stepwise processing and subcellular localization. The EMBO journal. 21, 4663-4670 (2002).
- Diederichs, S., Haber, D. A. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 131, 1097-1108 (2007).
- Perron, M. P., Provost, P. Protein interactions and complexes in human microRNA biogenesis and function. Frontiers in bioscience: A journal and virtual library. 13, 2537-2547 (2008).
- Wightman, B., Ha, I., Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 75, 855-862 (1993).
- Lee, R. C., Feinbaum, R. L., Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 75, 843-854 (1993).
- Kloosterman, W. P., Wienholds, E., Ketting, R. F., Plasterk, R. H. Substrate requirements for let-7 function in the developing zebrafish embryo. Nucleic acids research. 32, 6284-6291 (2004).
- Lee, I., et al. New class of microRNA targets containing simultaneous 5′-UTR and 3′-UTR interaction sites. Genome research. 19, 1175-1183 (2009).
- Djuranovic, S., Nahvi, A., Green, R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 336, 237-240 (2012).
- Iwakawa, H. O., Tomari, Y. The Functions of MicroRNAs: mRNA Decay and Translational Repression. Trends in cell biology. 25, 651-665 (2015).
- Wilczynska, A., Bushell, M. The complexity of miRNA-mediated repression. Cell deathand differentiation. 22, 22-33 (2015).
- Selbach, M., et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 455, 58-63 (2008).
- Kloosterman, W. P., Plasterk, R. H. The diverse functions of microRNAs in animal development and disease. Developmental cell. 11, 441-450 (2006).
- Mendell, J. T., Olson, E. N. MicroRNAs in stress signaling and human disease. Cell. 148, 1172-1187 (2012).
- Sayed, D., Abdellatif, M. MicroRNAs in development and disease. Physiologicalreviews. 91, 827-887 (2011).
- Steinkraus, B. R., Toegel, M., Fulga, T. A. Tiny giants of gene regulation: Experimental strategies for microRNA functional studies. Wiley interdisciplinary reviews. Developmental biology. 5, 311-362 (2016).
- Watanabe, Y., Tomita, M., Kanai, A. Computational methods for microRNA target prediction. Methods in enzymology. , 65-86 (2007).
- Lewis, B. P., Shih, I. H., Jones-Rhoades, M. W., Bartel, D. P., Burge, C. B. Prediction of mammalian microRNA targets. Cell. 115, 787-798 (2003).
- Baek, D., et al. The impact of microRNAs on protein output. Nature. 455, 64-71 (2008).
- Thomson, D. W., Bracken, C. P., Goodall, G. J. Experimental strategies for microRNA target identification. Nucleic acids research. 39, 6845-6853 (2011).
- Ebert, M. S., Neilson, J. R., Sharp, P. A. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nature. 4, 721-726 (2007).
- Elmen, J., et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic acids research. 36, 1153-1162 (2008).
- Jin, H. Y., et al. Transfection of microRNA mimics should be used with caution. Frontiersin genetics. 6, 340 (2015).
- Beitzinger, M., Peters, L., Zhu, J. Y., Kremmer, E., Meister, G. Identification of human microRNA targets from isolated argonaute protein complexes. RNA biology. 4, 76-84 (2007).
- Chi, S. W., Zang, J. B., Mele, A., Darnell, R. B. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 460, 479-486 (2009).
- Easow, G., Teleman, A. A., Cohen, S. M. Isolation of microRNA targets by miRNP immunopurification. RNA. 13, 1198-1204 (2007).
- Hafner, M., et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 141, 129-141 (2010).
- Cambronne, X. A., Shen, R., Auer, P. L., Goodman, R. H. Capturing microRNA targets using an RNA-induced silencing complex (RISC)-trap approach. Proceedings of theNational Academy of Sciences of the United States of America. , 20473-20478 (2012).
- Mili, S., Steitz, J. A. Evidence for reassociation of RNA-binding proteins after cell lysis: implications for the interpretation of immunoprecipitation analyses. RNA. 10, 1692-1694 (2004).
- Meister, G., et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Molecular cell. 15, 185-197 (2004).
- Su, H., Trombly, M. I., Chen, J., Wang, X. Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes & development. 23, 304-317 (2009).
- Wani, S., Cloonan, N. Profiling direct mRNA-microRNA interactions using synthetic biotinylated microRNA-duplexes. bioRxiv. , (2014).
- Grunweller, A., Hartmann, R. K. Locked nucleic acid oligonucleotides: The next generation of antisense agents?. BioDrugs: clinical immunotherapeutics,biopharmaceuticals and gene therapy. 21, 235-243 (2007).
- Guerard, M., et al. Locked nucleic acid (LNA): Based single-stranded oligonucleotides are not genotoxic. Environmental and molecular mutagenesis. 58, 112-121 (2017).
- Song, R., Ro, S., Yan, W. In situ hybridization detection of microRNAs. Methods inmolecular biology. , 287-294 (2010).
- Edbauer, D., et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 65, 373-384 (2010).
- Le, M. T., et al. MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Molecular and cellular biology. 29, 5290-5305 (2009).
- Dash, S., et al. Poly (ADP-Ribose) Polymerase-1 (PARP-1) induction by cocaine is post-transcriptionally regulated by miR-125b. eNeuro. 4, (2017).
- Micheli, F., et al. Regulation of proapoptotic proteins Bak1 and p53 by miR-125b in an experimental model of Alzheimer’s disease: Protective role of 17beta-estradiol. Neuroscience letters. 629, 234-240 (2016).
- Orom, U. A., Lund, A. H. Isolation of microRNA targets using biotinylated synthetic microRNAs. Methods. 43, 162-165 (2007).
- Cloonan, N. Re-thinking miRNA-mRNA interactions: Intertwining issues confound target discovery. BioEssays: News and reviews in molecular, cellular and developmental biology. 37, 379-388 (2015).
- Guo, Y. E., Steitz, J. A. 3′-Biotin-tagged microRNA-27 does not associate with Argonaute proteins in cells. RNA. 20, 985-988 (2014).
- Mook, O., et al. In vivo efficacy and off-target effects of locked nucleic acid (LNA) and unlocked nucleic acid (UNA) modified siRNA and small internally segmented interfering RNA (sisiRNA) in mice bearing human tumor xenografts. Artificial DNA, PNA & XNA. 1, 36-44 (2010).
- Owczarzy, R., You, Y., Groth, C. L., Tataurov, A. V. Stability and mismatch discrimination of locked nucleic acid-DNA duplexes. Biochemistry. 50, 9352-9367 (2011).

