Automatically Generated
Analysis of Circadian Photoresponses in Drosophila Using Locomotor Activity
Summary
Drosophila locomotor activity is a robust and quantitative measurement of circadian photo-responses. We describe protocols for designing behavior experiments for circadian photo-responses and analyzing the data. Studying the circadian photo-responses is important for dissecting the neuronal and molecular mechanisms of light entrainment.
Abstract
Circadian rhythms are only beneficial to animals if they can be synchronized by changes of ambient conditions. Light and temperature are two dominant environmental parameters that synchronize animal circadian clocks. In Drosophila circadian photo-responses are mediated by a solely blue light photoreceptor named CRYPTOCHROME (CRY). Upon photoreception, CRY changes its conformation and initiates the proteosomal dependent degradation of TIMELESS (TIM). TIM is an important pacemaker protein, thus degradation of TIM will reset the circadian clock. Under constant light conditions (LL), wild type flies quickly become arrhythmic because of the constant degradation of TIM, while flies bearing defects with circadian photo-responses will still be rhythmic. Thus LL triggered arrhythmicity has been used for screening of components in circadian light input pathways. A brief short light pulse in the night can also dramatically shift phases of circadian rhythms. As expected, this phase shift response is reduced in flied with defects in circadian photoresponse. Thus analyzing locomotion behavior rhythmicity under LL or phase changes after short light pulse in constant darkness (DD) are two major methods to study circadian photoresponse. Here we describe how to design and analyze LL and phase response experiments. LL arrhythmicity is suitable for screening light input pathways mutants, whereas phase response validates the results and provide further information for light sensitivity.
Introduction
Most organisms, from cyanobacteria to mammals, use circadian clocks to anticipate daily environmental changes. Circadian clocks synchronize most bodily functions of animals, from metabolic level, to rest/activity cycles and other behaviors1. Circadian rhythms are self-sustained, which can be maintained even in constant conditions for several days. Circadian rhythm is generated by a molecular pacemaker, which is highly conserved among organisms. In fruit flies, the core of this circadian clock, is a transcriptional-translational feedback loop2,3. Two transcription factors, CLOCK (CLK) and CYCLE (CYC) form a heterodimer and generate rhythmic transcriptions of down stream clock controlled genes. Among these genes, PERIOD (PER) and TIM are two main transcriptional repressors. PER/TIM undergo post-translational modifications, and accumulate in the cytoplasm, then enter the nucleus to repress their own transcription by blocking CLK activity.
Circadian pacemakers are self-sustained, but environmental cues determine their phase of oscillations. Light and temperature are the most crucial cues for synchronization of the circadian clock4. Compared to temperature, entrainment by light is much better understood. Circadian photo-responses are mainly mediated by CRY input pathways in flies. CRY is the blue light photoreceptor, which changes its conformation after receiving light, and then it is able to bind TIM5,6. After binding with CRY, TIM undergoes proteosomal dependent degradation through E3 ubiquitin ligase JETLAG7-9 (JET). Thus, the degradation of TIM resets the circadian pacemaker.
Flies become arrhythmic in constant light conditions, because of the constant degradation of TIM by CRY. However flies with defects in the CRY pathway still remain rhythmic. Based on this observation, the behavioral rhythmicity in LL is often used to demonstrate circadian photo-responses in flies. A short light pulse in the night will cause transient degradation of TIM, thus shifting the phase of circadian pacemaker5,6. Light pulse at early night will mimic a delayed day, thus called phase delay; while a light pulse at late night will mimic an advanced dawn, which is named as phase advance. This phase response is sensitive to light intensity, which is an important parameter for circadian photo-responses. Phase response is almost abolished in mutants of CRY input pathways. Measuring the phase response by light pulse is also extensively used to examine circadian photo-responses. Here we describe how to perform these two experiments as well as methods to analyze the behavior data.
Protocol
1. Constant light (LL) Experiments
- Preparation of experimental flies
- Raise flies in incubators or rooms that have regular light: dark cycles on standard fly food at 25°C. Collect female and male virgin flies for crosses.
NOTE: Unlike mated flies newly emerged virgin female flies have larger white abdomens. There is also a dark dot on their abdomen, which is only for virgin flies.). For RNAi screen, collect driver lines with GAL4 driven by circadianly regulated promoter (e.g. tim-GAL4 for all circadian neurons) and RNAi responder lines for crosses. - Put approximately 5 virgins and 2 males in a vial for each cross to ensure enough progenies for behavioral analysis. Collect cryb or other mutants in CRY input pathway as positive control.
- Eclosion takes around 14 days at room temperature. In 25°C incubator, it takes about 10 days for flies to eclose. Use CO2 to anesthetize flies and use small fine tipped paintbrush and collect 1-5 days old male progeny with right genotype on fly pad with CO2 flows for following LL behavior assay.
- Raise flies in incubators or rooms that have regular light: dark cycles on standard fly food at 25°C. Collect female and male virgin flies for crosses.
- Prepare fly activity tubes
- Autoclave glass activity tubes before use. Use 121°C, 29 psi for 45 minutes for autoclave. After autoclave, put activity tubes vertically into 500ml glass beaker before pouring the liquid fly food.
- Prepare white fly food for behavior (5% sucrose, and 2% bacto-agar). Heat deionized water with sucrose and bacto-agar and boil for at least 5 min. Pour liquid white food into beaker (1.2.1) to make approximately 1 cm food in each tube. Seal the activity tubes from the food side by plastic caps. Store the fly food in sealed box at 4°C.
- Set up LL experiment
NOTE: Before this experiment, make sure to have activity monitor systems set up as described10.- Load a single male fly using a small brush into each activity tubes. Seal the tube with cotton strips (about 0.5 cm long, made from regular cotton).
- Load activity tubes into activity monitors. Use at least 8 flies for each genotype. Write down the number (i.e 1-8, 9-16) for each genotype. Once inserted into the monitors, use rubber bands to prevent the activity tubes from falling out.
- Put the loaded activity monitor (such as DAM2) in incubators with light control. Connect the activity monitor to the data collection system with telephone wires.
- Set the incubator temperature at 25°C, 60% humidity, and light intensity around 1,000 lux.
- Use the activity monitor software to set the incubator light cycle as: 5 days of light:dark, and then 6 days constant light.
- LL experiment data analysis
- After the experiment, collect the raw data from the activity monitor software. Use activity monitor software to process the raw data. Sum the behavior data into 30 minute bins. Set the light on/off parameter as the same for the incubators.
- Use a spreadsheet program to assign different genotypes. Analyze the data with FaasX software (downloaded from F. Rouyer lab, CNRS, France). In FaasX, click "open experiment" in the main menu. From the "Fly group selection", choose the genotype to analyze, and name the fly group, then click "proceed".
- Then from the "Analysis" menu, choose "period, cycle_p" option. For analysis of rhythmicity in LL, choose the data from 5th day for 5 days. Define the rhythmic flies with following criteria: power 20, width 2.
NOTE: In certain circumstances, a power of 10 could also be used, it the robustness of the rhythm is not strong. Use a signal processing toolbox for a computing program such as Matlab to generate behavior Actograms. Wild type flies should show very low rhythmicity, flies with defects in circadian photoresponses show high rhythmicity in LL (50%-100%).
2. Phase responses Experiments
NOTE: The major part of preparation of this phase response experiment is the same as LL, except the behavior program and data analysis. Here we will only describe in detail the steps that diverge between the two experiments.
- Prepare experimental flies as described in Section 1.1 and 1.2.
- Load flies into activity tubes. Prepare at least 48 male flies for each genotype. Use 16 flies each for non-light pulse (NLP), light pulse at ZT15 (15hours after light on), and light pulse at ZT21 (21 hours after light on).
- Set up three sets of activity monitors with same genotypes and number of flies. Label monitors as NLP, ZT15, and ZT21.
- Use telephone wires to connect monitors with the data collection system. Put different sets of monitors onto different shelves in the incubator. Use a light meter to measure and set the light intensity at 1500 lux.
- Set incubator temperature at 25°C Set up the incubator light:dark cycles as: 5 days of light:dark, then 6 days of constant darkness.
- Perform light pulse on the last day of the light: dark cycle. Expose flies (set ZT15) to 1500 lux for 5 mins at ZT15 using light source or a separate incubator. Carefully put the monitors back and connect them. Repeat at ZT21. Perform this light pulse in the dark.
NOTE: 5 min light pulse is sufficient to shift the phase of circadian clock, and this acute phase shift is mediated by CRY pathway. - Collect data and transform with activity monitor software. Assign the genotypes and treatment in table. Analyze the data (from 2nd in DD for 5 days) with FaasX using the function of "Phase".
- Use two methods to calculate the phase shift. First, compare the "phase" differences of same genotype ZT15 and ZT21 versus NLP calculated in FaasX.
- In FaasX, click "open experiment" in the main menu. From the "Fly group selection", choose the genotype to analyze, and name the fly group, then click "proceed". Then from the "Analysis" menu, choose "phase" option.
- Choose the data from 1st day of constant darkness for 5 days for "Data Range". Check "at least through the data range requested" for "Fly survival". Define the phase point with "peak" or "valley". For the output, check "Phases" as "Text files"; check "Plot group mean waveform" for "Graphic".
- Then click "Run". Observe the FaasX generate a text file with periods and phases of each genotype in each treatment fly group. Compare the phase difference between ZT15 and NLP, as well as ZT21 and NLP.
NOTE: A brief light pulse in the early night mimics delayed dusk thus causes a phase delay, while light pulse at late night mimics advanced dawn and causes an advanced phase. Light pulse at ZT15 or ZT21 causes phase delay or advance. Use negative numbers to represent phase delay and positive number for phase advance. Sometimes, there are multiple peaks of activity; it becomes tricky to use the software. Then it is recommended to analyze the data by visual observation. - Repeat the same analysis except that for the "Graphic" option, check "Plot individual waveforms". Based on the waveforms, define the time at which the subjective night activity drops 50% as a "phase" marker for single fly of each genotype. Then compare the phase differences at both ZT15 and ZT21 with NLP and plot the phase changes.
Representative Results
Under constant light, wild type flies become arrhythmic because of constant degradation of TIM, while circadian photo-response mutants remain rhythmic. Figure 1 shows behavior actograms of flies under constant light. The results can also be presented in a quantitative manner. Table 1 shows the percentage of rhythmic flies, and period in constant light. Normally, very few wild type flies show rhythmicity (0-25%), while majority of flies with defects in circadian photo-response remains rhythmic (75-100%). By comparing the phase changes after light pulses to non light pulse control, phase responses are represented as "negative or positive" values separately (Figure 2). cry mutant flies have minimal phase responses, which are close to zero. If under LL, cry mutant flies are less than 75% rhythmic, usually that means the experiment fails. Make sure not to include dead flies in your analysis.
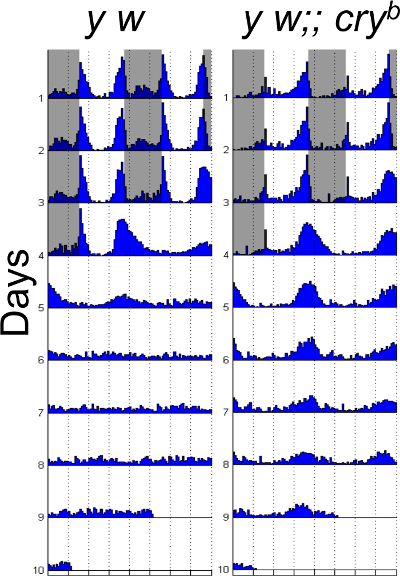
Figure 1: Locomotion behavior under constant light. Double plotted behavior actograms of y w control flies, and cryb mutants. Flies are entrained for three full days of light: dark (12:12hr), and then released into constant light for six days. cryb mutant flies are still rhythmic while wild type flies become arrhythmic. Grey area indicates dark phase. n=16 for each fly genotype. Please click here to view a larger version of this figure.
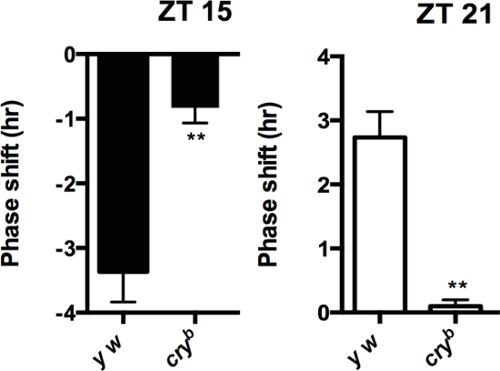
Figure 2: Phase responses after light pulse at ZT15 and ZT21. Flies are entrained for three days of standard light: dark cycles, and release into constant darkness for six days. A 5 min 1,500 lux light pulse is performed at ZT15 or ZT21 on the last night of light: dark cycle. Phase advance (left panel) and phase delay (right panel) are showed by negative and positive numbers compared to non light pulse on two separate graphs. Error bars represent SEM. Figure 1 and 2 are modified from Lamba et al. 2014 with permission. Please click here to view a larger version of this figure.
| Genotype | Number of flies (n) | % of rhythmic flies | Period average (±SEM) | Power average (±SEM) |
| y w | 32 | 3 (1/40) | 20.5 | 11.5 |
| cryb | 32 | 91 (29/32) | 23.9±0.11 | 63.2±4.49 |
Table 1:Percentage of Rhythmicity under constant light. Modified from Lamba et al. 2014 with permission.
Discussion
Circadian rhythms exist in most organisms on earth. Animals utilize circadian clocks to coordinate their bodily functions with daily changes. Since environmental conditions are variable, the circadian clock is only beneficial if it can be adjusted by different changes. For flies, light is the primary environmental cue used to synchronize and shift the circadian clock. Studying circadian photo-response is important for understanding how light is processed and regulates circadian behavior. Disruptions of circadian rhythms are also associated with depression, anxiety and many psychiatric disorders, such as bipolar disorder11.
Constant light and phase response behaviors are widely used to study circadian photo-responses. Recently, constant light has been successfully used to screen and identify genes involved in CRY input pathways12. It is an efficient, and reproducible method for behavior screening. Same as other behavioral assays, there is always some variability among individual flies.
It is important to have good sample size to get interpretable data. Based on experimental aims, the number of tested flies can be different. At least 8 flies are required for a LL screen, and 16 flies are suggested to confirm the results later. A brief light pulse in the night is sufficient to shift the circadian phase of fly behavior. ZT15 and ZT21 are the two typical time points to do light pulse, since they generate strong phase response. Pulsing with different light intensities, phase response experiments can also be useful for determining circadian photosensitivity. A 5 min 1500 lux pulse is sufficient to generate full response. 200 lux or even lower light pulse can be used to determine photosensitivity depending on different research purpose. For both LL and phase response experiments, light intensity and duration of light pulse are critical parameters.
Both constant light and light mediated phase responses are standard methods for measurement of circadian photoresponse. Constant light is suitable for screening, while phase response is typically used to delicately dissect the photo-response of a particular genotype. It is always useful to perform both of these experiments to validate circadian behavior phenotype.
Finally it is important to consider the genetic background of the strains when designing circadian photo-response experiments. In nature, there are two alleles of tim: s-tim and ls-tim, which have different light sensitivity13. Flies carrying s-tim allele is more sensitive to light, since s-TIM binds to CRY in a higher affinity way than ls-tim. It is recommended to check the tim allele of the strains when doing the circadian photo-responses experiments, thus to exclude background effects.The study of circadian photo-response may provide clues to investigate seasonal effects on sleep, mood and other psychiatric diseases.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103650, and the University of Nevada Reno. We thank Matthew Gruner and three anonymous reviewers for critically reading the manuscript and helpful comments.
Materials
| Percival Scientific | I-36LL | ||
| Trikinetics | DAM2 | activity monitor-2 | |
| Trikinetics | PGT5 | autoclavable | |
| Trikinetics | DAMSystem308 | free download | |
| Trikinetics | DAMFileScan110X | activity monitor-2 software | |
| Centre National de la Recherche Scientifique | Data analysis, Rouyer lab | ||
| Fishersci | S2-500GM | making fly food for behavior | |
| BD Biosciences | 214010 | making fly food for behavior |
References
- Mohawk, J.A., Green, CB, Takahashi, JS. Central and peripheral circadian clocks in mammals. Annu Rev Neuroscience. 35, 445- 462 (2012).
- Hardin, PE, Panda, S. Circadian timekeeping and output mechanisms in animals. Curr Opin Neurobiol. 5, 724-731 (2013).
- Zhang, Y, and Emery, P. Molecular and neural control of insect circadian rhythms. Insect Molecular Biology and Biochemistry. 15, 513-551, (2012).
- Dubruille, R, and Emery, P. A plastic clock: how circadian rhythms respond to environmental cues in Drosophila. Mol Neurobiol. 2, 129-145 (2008).
- Emery, P, So, WV, Kaneko, M, Hall, JC, Rosbash, M. CRY, A Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 95, 669-679 (1998).
- Stanewsky, R., et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 95, 681-692 (1998).
- Koh, K., Zheng, X., Seghal, A., JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 312, 1809-1812 (2006).
- Peschel, N, Chen, KF, Szabo, G, Stanewsky, R. Light-dependent interaction between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr Biol. 19, 241-237 (2009).
- Lamba, P, Bilodeau-Wentworth, D, Emery, P, Zhang, Y. Morning and evening oscillators cooperate to reset circadian behavior in response to light input. Cell Rep. 7, 601-608 (2014).
- Chiu, JC, Low, KH, Pike, DH, Yildirim, E, Edery, I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J Vis Exp. 43. Pii:2157 (2010).
- Abreu, T, Bragança, M. The bipolarity of light and dark: A review on Bipolar Disorder and circadian cycles. J Affect Disord. 185:219-29. (2015).
- Dubruille, R, Murad, A, Rosbash, M, Emery, P. A constant light-genetic screen identifies KISMET as a regulator of circadian photoresponses. PloS Genet. (2009).
- Peschel, N., Veleri, S., Stanewsky R. Veela defines a molecular link between Cryptochrome and Timeless in the light-input pathway to Drosophila's circadian clock. Proc Natl Acad Sci USA. 103, 17313-17318 (2006).

.
