Covalent Binding of BMP-2 on Surfaces Using a Self-assembled Monolayer Approach
Summary
We describe a method for accomplishing efficient immobilization of BMP-2 on surfaces. Our approach is based on the formation of a self-assembled monolayer to achieve the covalent binding of BMP-2 via its free amine residues. This method is a useful tool to study signaling at the cell membrane.
Abstract
Bone morphogenetic protein 2 (BMP-2) is a growth factor embedded in the extracellular matrix of bone tissue. BMP-2 acts as trigger of mesenchymal cell differentiation into osteoblasts, thus stimulating healing and de novo bone formation. The clinical use of recombinant human BMP-2 (rhBMP-2) in conjunction with scaffolds has raised recent controversies, based on the mode of presentation and the amount to be delivered. The protocol presented here provides a simple and efficient way to deliver BMP-2 for in vitro studies on cells. We describe how to form a self-assembled monolayer consisting of a heterobifunctional linker, and show the subsequent binding step to obtain covalent immobilization of rhBMP-2. With this approach it is possible to achieve a sustained presentation of BMP-2 while maintaining the biological activity of the protein. In fact, the surface immobilization of BMP-2 allows targeted investigations by preventing unspecific adsorption, while reducing the amount of growth factor and, most notably, hindering uncontrolled release from the surface. Both short- and long-term signaling events triggered by BMP-2 are taking place when cells are exposed to surfaces presenting covalently immobilized rhBMP-2, making this approach suitable for in vitro studies on cell responses to BMP-2 stimulation.
Introduction
Bone morphogenetic protein 2 (BMP-2) is a member of the transforming growth factor (TGF-β) family and acts as inducer of de novo bone formation as well as regulator of several tissues during embryonic development and adult homeostasis1-3. Each monomer of the biologically active homodimeric BMP-2 protein contains a "cysteine knot" motif, which is highly conserved in all BMPs4. Six of the seven cysteine residues form intramolecular disulfide bonds that stabilize each monomer, whereas the seventh cysteine is involved in dimerization, forming an intermolecular bond between the two monomers5,6. This highly conserved cysteine knot defines the three-dimensional structure of the BMP-2 protein and determines its unique properties, such as resistance against heat, denaturants and acidic pH7-9. BMP-2 binds to serine/threonine kinase transmembrane receptors, thereby inducing signal transduction10-12. Depending on the mode of receptor oligomerization, different signaling pathways are activated: a Smad-independent signaling cascade leads to alkaline phosphatase induction via p38 signaling, whereas a Smad-dependent pathway activated by receptor phosphorylation results in Smad complex nuclear translocation and activation of transcription of specific target genes, such as the inhibitor of differentiation (Id)12-14.
In bone, BMP-2 induces the differentiation of mesenchymal stem cells into osteoblasts, thus stimulating the healing and de novo formation of bone. Currently, recombinantly expressed BMP-2 is applied clinically to enhance the healing of fractured sites. A common strategy in bone tissue engineering is the use of injectable growth factors, which is less invasive compared to local delivery systems. However, in vivo studies and clinical applications have shown that the short biological half-life, unspecific localization and rapid local clearance of BMP-2 may lead to several local, ectopic and systemic problems15. Hence, to obtain an effective presentation, the entrapment or immobilization of BMP-2 within or onto materials is necessary for its local and sustained delivery at the target site. Sustained delivery can be achieved with non-covalent retention approaches, such as physical entrapment, adsorption or ion complexation16. However, it is known that unspecific adsorption of proteins to surfaces may results in denaturation of the molecules17. For the covalent binding of growth factors, different types of supports have been developed over the last decade. The use of bifunctional linking molecules that target amino or carboxyl groups of the protein for example, is one type of approach that does not necessarily require protein modification to achieve its immobilization. In fact, while protein modification offers the advantage of controlling protein orientation, the introduction of artificial domains, peptide tags and site-specific chains may alter the biological activity of growth factors17. Thus, to circumvent denaturation due to interaction with the supporting material, surfaces can be functionalized beforehand, for example, with a self-assembled monolayer (SAM) of a linking molecule, followed by coupling of the desired factor18. We have used a SAM-based approach to covalently immobilize BMP-2 onto a surface by targeting its free amine residues and have shown that the immobilized protein retains both its short- and long-term biological activity19. This protocol provides a simple and efficient way to deliver BMP-2 to cells for in vitro studies on the mechanisms which occur at the cell membrane and regulate intracellular signaling responsible for osteogenic signaling.
Protocol
1. Synthesis of 11-Mercaptoundecanoyl-N-hydroxysuccinimide Ester (MU-NHS)
- Add dropwise a solution of 500 mg N-hydroxysuccinimide and 30 mg 4-(dimethylamino)pyridine in 10 ml acetone (p.a.) to 1 g 11-mercaptoundecanoic acid in 40 ml dichloromethane (p.a.) at room temperature (RT).
- Cool the reaction to 0 °C and add dropwise 1.1 g N,N'-dicyclohexylcarbodiimide in 10 ml dichloromethane (under nitrogen atmosphere). Keep the reaction at low temperature for 1 hr and then stir at RT overnight.
- Filter the precipitate and dry it under reduced pressure. Purify the product by flash chromatography with petroleum benzene and ethyl acetate (p.a.) in a ratio of 1:1.
2. Preparation of Homogeneous Gold Layers
- Clean glass coverslips with precision wipes and sonicate them for 5 min in a solution containing a 1:1 mixture of ethyl acetate (p.a.) and methanol (p.a.). Rinse substrates with methanol and dry off under nitrogen flow.
- Place the clean substrates in the sputter coating device. Evacuate the chamber. Remove the uppermost chromium oxide layer from chromium target by sputtering for 60 sec. Place the samples underneath the metal beam and coat them with a 10 nm chromium layer, followed by coating with a 40 nm gold layer.
3. Surface Immobilization of BMP-2
- Dissolve MU-NHS in N,N-dimethlyformamide (DMF) to a final concentration of approximately 1 mM and incubate gold substrates in the MU-NHS solution at RT for 4 hr under nitrogen atmosphere.
- Sonicate surfaces in DMF for 2 min, rinse with DMF and MeOH and dry off under nitrogen flow.
- Prepare a stock solution of rhBMP-2 in sterile 4 mM HCl with a concentration of 100 μg/ml (store aliquots at -80 °C). For working dilutions (3.5 μg/ml), dilute the stock solution in PBS/NaCl (PBS containing 1 M NaCl) and adjust to pH 8.5 immediately prior to use.
- Incubate surfaces functionalized MU-NHS in the rhBMP-2 working solution at 4 °C overnight. Remove incubation supernatant. Sonicate surfaces in PBS/NaCl for 2 min and wash 3x with sterile PBS/NaCl.
4. Surface Characterization
- To block unspecific adsorption of antibody, incubate surfaces with a 5% BSA (w/v) in PBS solution for 1 hr at RT, followed by incubation with an anti-BMP-2 antibody (1:100 in 1% (w/v) BSA/PBS solution) for 1 hr at RT. Wash surfaces twice with PBS and sonicate for 30 sec.
- Incubate substrates with HRP-conjugated secondary antibody (1:1,000 in 1% (w/v) BSA/PBS solution) for 30 min at RT, then wash twice with PBS.
- Use Ampliflu Red assay to measure HRP enzymatic activity at 570 nm with a plate reader.
5. Analysis of Biological Activity
- Seed 1 x 105 mouse C2C12 myoblasts per well in 6-well plates in growth medium, consisting of a high glucose DMEM containing pyruvate supplemented with 10% FBS, 1% penicillin/streptomycin and incubate them for 24 hr at 37 °C/5% CO2.
- Starve cells in serum-free DMEM for 3-5 hr prior to seeding onto the surfaces decorated with immobilized BMP-2.
- For the investigation of short-term signaling induction, replace the medium by 200 μl fresh serum-free DMEM and place the immobilized BMP-2 surfaces above the cells. The surface should be handled with fine tip tweezers to avoid scratching.
- Remove gently surfaces and aspirate the medium with a pipette, wash the cells twice with PBS. Proceed to analysis of cell responses.
- For the analysis of long-term biological activity, plate cells onto surfaces and culture them at 37 °C/5% CO2 in low serum conditions (2% FBS) for six days.
Representative Results
In our setup, gold was chosen as excipient since it provides a biologically unspecific but chemically tunable system. Furthermore, the application of self-assembling monolayers entails many benefits: SAMs spontaneously adsorb via their “head-groups” on metals and form monolayers with few defects, while their functional end-groups can be further modified. Thus they provide a platform to tailor the properties of the interface in a controlled yet highly adaptable way20.
For the immobilization of BMP-2 on gold-coated surfaces, we used a two-step approach: 1) binding the MU-NHS linker to the gold layer, and then 2) reacting the linker with the protein (see Figure 1). To prove the binding of the rhBMP-2, we used an enzyme immunoassay. In this assay, surfaces presenting immobilized rhBMP-2 were incubated with a BMP-2 specific antibody and a secondary antibody conjugated with HRP. The binding of the latter can be determined using an Ampliflu Red solution. In the presence of hydrogen peroxide, HRP catalyzes the conversion of the colorless Ampliflu Red (10-acetyl-3,7-dihydroxyphenoxazine) into the fluorescent compound resorufin. We detected surface immobilized rhBMP-2 (iBMP-2) before and after approaching adherent cells from above with functionalized surfaces. Please note that for the detection of rhBMP with the Ampliflu Red assay, the samples are subject to several incubation steps and treatments. Therefore, it is not feasible to investigate the same surface before and after cell stimulation. Figure 2A shows the successful binding of the rhBMP-2 to the surface in a conformation recognized by the anti-BMP-2 IgG. Figure 2B shows an iBMP-2 surface that was used to stimulate cells for 30 min prior to the HRP immunoassay. Even after cell stimulation the protein is detected on the surface.
Cell responses to surfaces presenting immobilized rhBMP-2 were evaluated and compared to the effect of non-treated gold substrates (negative control). In order to minimize the influence of the adhesion process on the analysis of the short-term signaling, a special set-up was developed for cell stimulation. Here, C2C12 cells were stimulated for 30 min by rhBMP-2 functionalized surfaces approaching from the top. The mouse myoblast cell line C2C12 is a pluripotent mesenchymal precursor cell line, which is widely used as a model system to study the early stage of osteogenic differentiation during bone formation in muscular tissues. In this model, BMP-2 inhibits the differentiation of cells into multinucleated myotubes and induces osteoblast phenotypes21. The activation of Smad 1/5/8, which are downstream reporters of the BMP-2 signaling pathway, is analyzed by western blotting. As shown in Figure 3A, Smad phosphorylation is observed after 30-min stimulation by iBMP-2, while no phosphorylation of Smad 1/5/8 occurs in cells exposed to untreated gold samples. This result indicates that the immobilization process does not alter BMP-2 short-term activity and triggers early steps in Smad signaling.
To determine whether iBMP-2 affects long-term osteogenic differentiation, the expression level of alkaline phosphatase (ALP), an osteogenic marker, was investigated by a colorimetric assay. ALP activity of C2C12 cells was measured after incubating the cells for 6 days on plain gold (control) and iBMP-2 surfaces. ALP activity in lysates of cells cultured on iBMP-2 surfaces shows a significantly higher absorption in comparison to the control (Figure 3B). This result reveals that iBMP-2 induces the expression of the osteogenic marker ALP in C2C12 cells. This cell line is known to differentiate upon reaching confluency under low serum condition, thereby forming myotubes and expressing characteristic myogenic proteins. However, treatment with BMP-2 causes a shift in the differentiation pathway from myoblastic to osteoblastic, therefore suppressing the formation of myotubes. To investigate the effect of iBMP-2 on myogenesis, C2C12 cells were plated directly on gold (control) or functionalized surfaces and cultured under differentiation (low serum) conditions. After 6 days, staining of myosin heavy chain (MHC) was performed. As shown in Figure 3C, C2C12 cells fail to form MHC positive myotubes in presence of iBMP-2, but not on gold substrates.
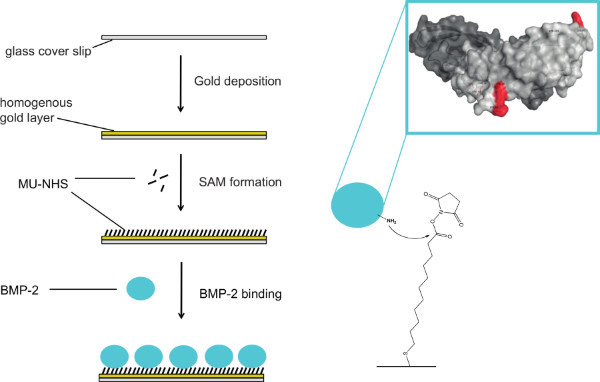
Figure 1. Scheme of the immobilization process of rhBMP-2 onto gold surfaces. Glass coverslips are coated with a gold layer and subsequently incubated with a heterobifunctional linker (MU-NHS) resulting in a NHS-functionalized self-assembled monolayer (SAM). Primary amines of BMP-2 react with the linker leading to covalently immobilized protein on the substrate. Click here to view larger figure.
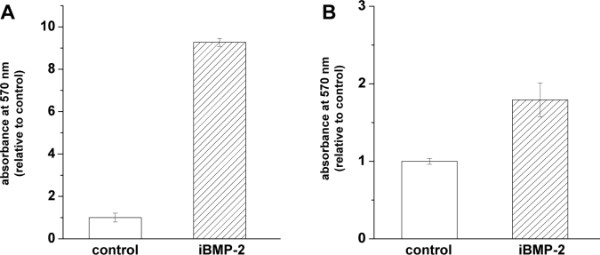
Figure 2. Surface-bound rhBMP-2 can be detected by enzymatic immunoassay before (A) as well as after (B) cell stimulation experiments. Immobilized rhBMP-2 was quantified by using Ampliflu Red colorimetric assay. The absorption was measured at 570 nm and data were normalized to control (non-treated gold surfaces) values. Error bars represent the standard deviation from the mean n>3.
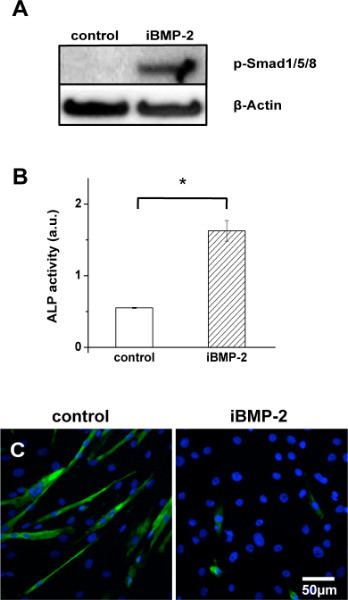
Figure 3. Immobilized rhBMP-2 remains biologically active and induces short-term as well as long-term signaling events. (A) C2C12 cells were stimulated for 30 min by exposure to gold surfaces (control) and surfaces with covalently immobilized rhBMP-2 (iBMP-2) approaching from the top. After cell lysis, samples were immunoblotted for p-Smad1/5/8 and β-actin. (B) The enzymatic activity of alkaline phosphatase (ALP) indicates osteogenic differentiation of C2C12 cells and was measured from cell lysates after a 6-day incubation period on control or iBMP-2 surfaces, respectively. ALP activity was measured as absorbance at 405 nm. Bar graph shows data acquired after a 60-min reaction. The error bars indicate standard deviation, n=3, *p<0.01. (C) C2C12 cells were seeded on indicated surfaces and cultured for 6 days under low serum conditions to allow myogenesis. Images show myosin heavy chain (MHC) staining of multinucleated myotubes (green) and DAPI nuclei staining (blue). Image magnification 20x.
Discussion
In this protocol we describe the preparation of surfaces functionalized with bioactive rhBMP-2. This approach comprises two steps: 1) the initial formation of a self-assembling monolayer (SAM) of a bifunctional linker on the gold surface; 2) covalent immobilization of the rhBMP-2 protein. In previous work, we validated the effective binding of the bifunctional linker and the growth factor, and demonstrated that surface-immobilized rhBMP-2 maintains its biological activity19. The bioactivity of growth factors presented in an immobilized form has also been shown in other studies, indicating that the release of the proteins and their subsequent internalization are not essential for cell stimulation24,25. In fact, the covalent immobilization of growth factors allows the sustained stimulation of the target cells while maintaining an effective dosage because of enhanced occupancy rate of different types of receptors at the cell membrane 22,23,26-28.
For the preparation of surfaces presenting immobilized rhBMP-2, care should be taken in handling the surfaces throughout the whole procedure and in preparing the rhBMP-2 solution. The glass coverslips should be clean and dry and no signs of defect or impurities should be visible. Scratching the surface with tweezers and pipettes should be avoided. For the surface functionalization step using rhBMP-2, it is essential to use a carrier-free recombinant protein, i.e. without added bovine serum albumin in the preparation, to avoid undesired immobilization of the carrier on the surface. The rhBMP-2 stock (100 μg/ml) is dissolved in 4 mM HCl. When surfaces are incubated with the rhBMP-2 solution, the pH should be adjusted to neutral to slight alkaline pH to enhance the reaction of the amino group of the protein with the NHS group of the surface-bound linker. If the pH is too low, the NHS group might be hydrolyzed before it can react with the amino groups of the protein. We use PBS containing 1 M NaCl29 and add rhBMP-2 from stock and then adjust the pH with KOH (10 mM).
With this protocol we achieved the successful immobilization of bioactive rhBMP-2 on surfaces because: 1) we established a two-step approach; 2) we used an appropriate linker to obtain distance from the surface and to bind the protein without major interferences on its interaction with the receptor. The amine-reactive alkanethiol, 11-mercaptoundecanoyl-N-hydroxysuccinimide ester (MU-NHS), was tethered to the gold substrate in the first step. As alkanethiols self-assemble on metals like gold, forming a highly ordered monolayer, they facilitate the decoration of surfaces with a variety of linker molecules30-32. The SAM shields biomolecules from direct contact with solid surface, thereby minimizing the risk of denaturation32. Furthermore, the length of the linker also determines the reactivity of the SAM. The NHS groups of linkers consisting of at least eleven carbon atoms, like the MU-NHS, are more accessible, resulting in increased protein immobilization compared to binding to shorter linkers34. The immobilization of rhBMP-2 occurs through the displacement of the NHS group by the lysine residues of the protein34. Considering merely the steric environment, the reaction of the surface-immobilized NHS groups most likely takes place at the flexible N-terminal residues35. However, as shown for other ligands, the length and the flexibility of the linker might enable interaction with otherwise sterically hindered binding sites, thus compensating for unfavorable orientation36,37. In unpublished work we compared the one-step with a two-step immobilization strategy. The comparison between using a solution containing both protein and linkers, and the sequential surface binding of the linker and the protein, showed that only the two-step strategy resulted in bioactive ligand immobilization. This might be due to a shielding effect of the SAM, which hinders protein denaturation that could otherwise take place on gold. Furthermore, since the linker is already anchored to the surface, cross-linkage between proteins through the end groups of the linker and their resulting inactivation are circumvented38.
For the experiments with cells stimulated with surface-immobilized rhBMP-2, it is crucial to determine beforehand that C2C12 did not form myotubes in the culture flask prior to seeding them onto the substrate. Therefore, cells have to be kept subconfluent in culture. The batch of fetal bovine serum used for culturing and for the experiments has to be tested to ensure that no osteogenic stimuli are present. This is performed by using the Quantikine assay for detection of bone morphogenetic proteins and by testing the cells for osteogenic differentiation markers, e.g. alkaline phosphatase expression. When handling the substrates for the stimulation from the top of cultures, it is again important to avoid any scratches with the tweezers. Drying of the culture and squeezing of the cells should be avoided in any case, otherwise cell distortion and death will negatively affect the response to BMP-2 stimulation. We recommend pipetting approximately 100 μl of DMEM into each well of a 6-well plate where the cells are cultured before applying the substrate onto the cells. Cells should be observed under the microscope to assure that no morphological changes are triggered by the presence of the surfaces on top of the cultures. Finally, when removing the substrates, one side is carefully lifted with the tweezers and the substrate is gently peeled off. An immediate check for any cell damage with a bright field microscope should be performed, as well as a check of the substrates to determine if any cell tethers or major impurities are present.
In conclusion, the presented two-step procedure provides a useful tool to immobilize rhBMP-2 onto substrates via its amine residues to study its impact on cellular responses. The described strategy combines many advantages. On the one hand, it is not restricted to BMP-2, but it can be also applied to other growth factors of the BMP family, since they present a highly conserved amino acid structure. On the other hand, by preventing unspecific adsorption, this approach enables targeted investigation of BMP-2, while reducing the amount of growth factor and, most notably, hindering uncontrolled release from the surface.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Prof. J.P. Spatz (Department of Biophysical Chemistry, University of Heidelberg and Department of New Materials and Biosystems, Max Planck Institute for Intelligent Systems, Stuttgart) for his kind support. The financial support from the Max-Planck-Gesellschaft and the Deutsche Forschungsgemeinschaft (DFG SFB/TR79 to E.A.C.-A.) are also greatly acknowledged.
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| N-hydroxysuccinimide | Sigma-Aldrich | 130672 | |
| 4-(dimethylamino)pyridin | Sigma-Aldrich | 522805 | |
| Acetone | AppliChem | A2282 | |
| 11-mercaptoundecanoic acid | Sigma-Aldrich | 674427 | |
| Dichlormethane | Merck | 106050 | |
| N,N'-dicyclohexylcarbodiimide | Sigma-Aldrich | D80002 | |
| Petroleum benzene | Merck | ||
| Glass coverslips | Carl Roth | M 875 | |
| Ethylacetate | AppliChem | A3550 | |
| Methanol | Carl Roth | 4627 | |
| N,N-dimethylformamide | Carl Roth | T921 | |
| rhBMP-2 | R&D Systems | 355-BM | Carrier-free; expressed in E.coli |
| PBS | PAA | H15-002 | |
| NaCl | Carl Roth | HN00.2 | |
| Poly(dimethyl siloxane) (PDMS) | Dow Corning | ||
| Sylgard 184 silicone elastomer kit | Dow Corning | ||
| Anti-rhBMP-2 | Sigma | B9553 | |
| Goat anti-mouse IgG-HRP | Santa Cruz | sc-2005 | Secondary antibody |
| Ampliflu Red assay | Sigma | 90101 | |
| Dulbecco's Modified Eagle Medium (DMEM) (1x), liquid | Gibco | 41966 | High glucose |
| Fetal Bovine Serum (FBS) | Sigma | F7524 | Sterile filtered, cell culture tested |
| Pen/Strep | Gibco | 15140 | |
| Trypsin 0.05% (1x) with EDTA 4Na | Gibco | 25300 | |
| Glycine (0.1 M) | Riedel-de Haën | 33226 | |
| IGEPAL CA-630 (1%) | Sigma | I8896 | Lysis buffer (ALP assay)19 |
| Magnesium chloride (MgCl2)(1 mM) | Carl Roth | HNO3.2 | |
| Zinc chloride (ZnCl2) (1 mM) | Carl Roth | 3533.1 | |
| p-nitrophenylphosphate (pNPP) | Sigma | S0942 | Phosphatase substrate |
| Anti-mysin heavy chain (MHC) | Developmental Studies Hybridoma Bank, University of Iowa | MF20 | Monoclonal antibody |
| Alexa Fluor 488 Goat anti-mouse IgG | Invitrogen | A11001 | |
| DAPI | Sigma | D9542 | |
| Equipment | |||
| Ultrsonic bath (Sonorex Super RK 102H), Frequency 35 kHz | BANDELIN electronic GmbH & Co. KG | ||
| MED 020 Sputtercoating system | BAL-TEC AG | Coating conditions Cr: 120 mA, 1.3 x 10-2 mbar, 30 sec Au: 60 mA, 5.0 x 10-2 mbar, 45 sec |
|
| Tecan Infinite M200 Plate reader | Tecan |
References
- Helm, G., Andersson, D., et al. Summary statement: Bone morphogenetic proteins: Basic science. Spine. 27 (16S), S9 (2002).
- Hogan, B. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10 (13), 1580-1594 (1996).
- Reddi, A. BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 16, 249-250 (2005).
- Rengachary, S. Bone morphogenetic proteins: basic concepts. Neurosurg Focus. 13 (6), 1-6 (2002).
- Schlunegger, M., Grütter, M. An unusual feature revealed by the crystal structure at 2.2 Å; resolution of human transforming growth factor-β2. Nature. 358, 430-434 (1992).
- Scheufler, C., Sebald, W., Hülsmeyer, M. Crystal structure of human bone morphogenetic protein-2 at 2.7 Å resolution. J. Mol. Biol. 287 (1), 103-115 (1999).
- Nimni, M. Polypeptide growth factors: targeted delivery systems. Biomaterials. 18 (18), 1201-1225 (1997).
- Wozney, J., Rosen, V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin. Orthop. Related Res. 346, 26 (1998).
- Rosen, V. BMP and BMP inhibitors in bone. Annals of the New York Academy of Sciences. 1068, 19-25 (2006).
- Kirsch, T., Sebald, W., Dreyer, M. K. Crystal structure of the BMP-2-BRIA ectodomain complex. Nat. Struct. Biol. 7 (6), 492-496 (2000).
- Keller, S., Nickel, J., et al. Molecular recognition of BMP-2 and BMP receptor IA. Nat. Struct. Mol. Biol. 11 (5), 481-488 (2004).
- Miyazono, K., Maeda, S., Imamura, T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 16 (3), 251-263 (2005).
- Nohe, A., Hassel, S., et al. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 277 (7), 5330-5338 (2002).
- Sieber, C., Kopf, J., et al. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 20 (5-6), 343-355 (2009).
- Carragee, E. J., Hurwitz, E. L., Weiner, B. K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 11, 471-491 (2011).
- Luginbuehl, V., Meinel, L., et al. Localized delivery of growth factors for bone repair. Eur. J. Pharm. Biopharm. 58 (2), 197-208 (2004).
- Nakaji-Hirabayashi, T., Kato, K., et al. Oriented immobilization of epidermal growth factor onto culture substrates for the selective expansion of neural stem cells. Biomaterials. 28 (24), 3517-3529 (2007).
- Gonçalves, R., Martins, M., et al. Bioactivity of immobilized EGF on self-assembled monolayers: Optimization of the immobilization process. J. Biomed. Mater. Res. Part A. 94A. 2 (2), 576-585 (2010).
- Pohl, T. L. M., Boergermann, J. H., et al. Surface immobilization of bone morphogenetic protein 2 via a self-assembled monolayer formation induces cell differentiation. Acta Biomater. 8 (2), 772-780 (2012).
- Love, J., Estroff, L., et al. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105 (4), 1103-1169 (2005).
- Katagiri, T., Yamaguchi, A., et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 127 (6), 1755-1766 (1994).
- Whitaker, M. J., Quirk, R. A., et al. Growth factor release from tissue engineering scaffolds. J. Pharm. Pharmacol. 53 (11), 1427-1437 (2001).
- Uludag, H., D’Augusta, D., et al. Implantation of recombinant human bone morphogenetic proteins with biomaterial carriers: a correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J. Biomed. Mater. Res. 50 (2), 227-238 (2000).
- Kashiwagi, K., Tsuji, T., et al. Directional BMP-2 for functionalization of titanium surfaces. Biomaterials. 30 (6), 1166-1175 (2008).
- Karageorgiou, V., Meinel, V. L., et al. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. 71 (3), 528-573 (2004).
- Rose, F. R. A. J., Hou, Q., et al. Delivery systems for bone growth factors – the new players in skeletal regeneration. J. Pharm. Pharmacol. 56 (4), 415-427 (2004).
- Masters, K. S. Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromol. Biosci. 11 (9), 1149-1163 (2011).
- Crouzier, T., Fourel, L., et al. Presentation of BMP-2 from a soft biopolymeric film unveils its activity on cell adhesion and migration. Adv. Mater. 23 (12), H111-H118 (2011).
- Ruppert, R., Hoffmann, E., et al. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. European Journal of Biochemistry. 237 (1), 295-302 (1996).
- Love, J., Estroff, L., et al. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105 (4), 1103-1169 (2005).
- Kato, K., Sato, H., Iwata, H. Immobilization of histidine-tagged recombinant proteins onto micropatterned surfaces for cell-based functional assays. Langmuir. 21 (16), 7071-7075 (2005).
- Martins, M., Curtin, S., et al. Molecularly designed surfaces for blood deheparinization using an immobilized heparin-binding peptide. J. Biomed. Mater. Res. 88 (1), 162-173 (2009).
- Limbut, W., Kanatharana, P., et al. A comparative study of capacitive immunosensors based on self-assembled monolayers formed from thiourea, thioctic acid, and 3- mercaptopropionic acid. Biosens. Bioelectron. 22 (2), 233-240 (2006).
- Patel, N., Davies, M., et al. Immobilization of protein molecules onto homogeneous and mixed carboxylate-terminated self-assembled monolayers. Langmuir. 13 (24), 6485-6490 (1997).
- Hu, J., Duppatla, V., et al. Site-specific PEGylation of bone morphogenetic protein-2 cysteine analogues. Bioconjug. Chem. 21 (10), 1762-1772 (2010).
- Hersel, U., Dahmen, C., Kessler, H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 24 (24), 4385-4415 (2003).
- Cao, T., Wang, A., et al. Investigation of spacer length effect on immobilized Escherichia coli pili-antibody molecular recognition by AFM. Biotechnol. Bioeng. 98 (6), 1109-1122 (2007).
- Puleo, D., Kissling, R., Sheu, M. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials. 23 (9), 2079-2087 (2002).

