Obtaining a Single-Cell Suspension from Mouse Hippocampal Tissue
Abstract
Source: Trujillo, M., et al. Combined Mechanical and Enzymatic Dissociation of Mouse Brain Hippocampal Tissue. J. Vis. Exp. (2021)
This video demonstrates a protocol to obtain a highly viable single-cell suspension from the mouse brain hippocampus using a combination of automated mechanical dissociation and a mixture of enzymes to aid in enzymatic digestion.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Prepare Enzyme Mix 1 and 2 for each sample
NOTE: For volumes greater than 2 mL, use a 10 mL serologic pipette; for volumes, 200 µL-2 mL, use a 1000 µL pipette; for volumes, 21-199 µL, use a 200 µL pipette; for volumes, 2-20 µL, use a 20 µL pipette; for volumes under 2 µL, use a 0-2 µL pipette.
- For each sample, thaw one aliquot each of Enzyme P and Enzyme A at room temperature.
- For Enzyme mix 1, combine 50 µL of Enzyme P and 1900 µL of Buffer Z in a labeled C Tube (Table of Materials).
- For Enzyme mix 2, add 20 µL of Buffer Y to the thawed 10 µL aliquot of Enzyme A.
2. Adult brain dissociation protocol
NOTE: When working with samples, tubes should be placed in a tube rack at room temperature while BSA and D-PBS remain on ice unless otherwise noted.
- Switch on the dissociator.
- Use forceps to transfer the hippocampi tissue pieces to the C Tube.
- Transfer 30 µL of Enzyme mix 2 into the C Tube. Twist the cap until tension is felt, then tighten until it clicks.
- Place the C Tube upside down into a position of the dissociator; the sample will be assigned the Selected status (Figure 1). Secure the heater over the C Tube.
- Press the folder icon, select Favorites folder, scroll to and select the 37C_ABDK_02 program. Click on OK to apply the program to all selected C tubes, then tap on Start (Figure 1).
- Label one 50 mL conical tube per sample.
- Place a 70 µm cell strainer on each 50 mL conical tube and wet with 2 mL of BSA buffer.
- Upon completion of the program, remove the heater and the C tube from the dissociator.
- Add 4 mL of BSA buffer to the sample and apply the mixture to the cell strainer on the 50 mL conical tube.
- Add 10 mL of D-PBS to the C Tube, close it, and swirl the solution gently. Apply it to the cell strainer on the 50 mL conical tube.
- Discard the cell strainer and the C Tube. Centrifuge the suspension at 300 x g for 10 min at 4°C. Then, aspirate and discard the supernatant.
3. Debris removal
- Resuspend the pellet with 1550 µL of cold D-PBS and transfer the suspension to a labeled 15 mL conical tube.
- Add 450 µL of cold Debris Removal Solution and pipette up and down (do not vortex).
NOTE: Debris Removal Solution is a reagent in the commercial Adult Brian Dissociation Kit. - Gently overlay 1 mL of cold D-PBS on top of the cell suspension, keeping the tip against the wall of the conical tube. Repeat until the total overlay is 2 mL.
NOTE: This step is critical; perform with proficiency and dexterity. - Centrifuge at 3000 x g for 10 min at 4°C with full acceleration and full brake.
NOTE: If the phases are not clearly separated, repeat steps 7.2-7.3. Centrifuge a final time at 1000 x g for 10 min at 4°C. - The suspension should now consist of three distinct layers (Figure 2). Aspirate the topmost layer. Sweep the pipette tip back and forth to aspirate the white middle layer. Remove as much of the middle layer as possible without disturbing the bottom-most layer.
NOTE: This step is critical; perform with proficiency and dexterity. - Add 2 mL of cold D-PBS and pipette up and down to mix.
- Centrifuge at 1000 x g for 10 min at 4°C with full acceleration and full brake. Aspirate and discard the supernatant. Resuspend the pellet in 1 mL of BSA buffer.
NOTE: Cells can be resuspended in the appropriate buffer then magnetically labeled and isolated in preparation for single-cell sequencing at this point.
Representative Results
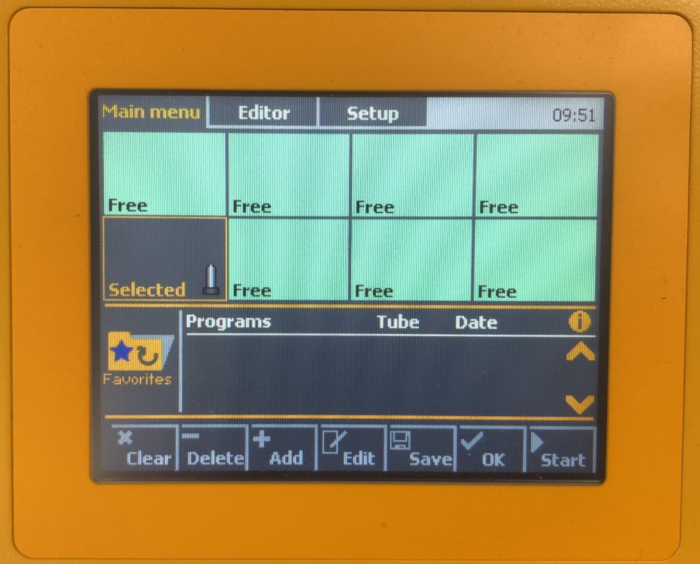
Figure 1: Selected status in step 2.4
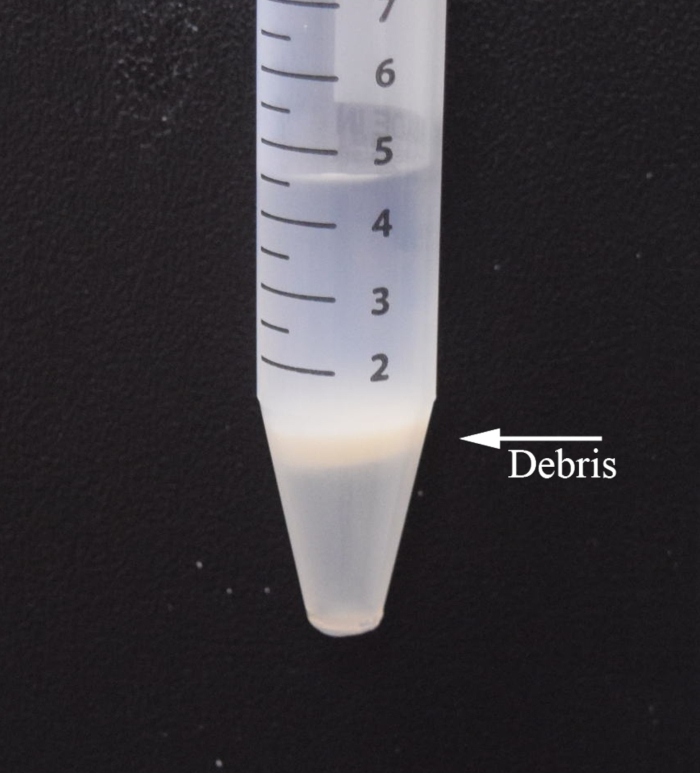
Figure 2: Tri-layered suspension in step 3.5: Buffer (top layer), cell debris, debris removal solution, and cells (bottom layer).
Disclosures
The authors have nothing to disclose.
Materials
| 1.5 mL Microcentrifuge Tubes | Fisher Scientific | 02-682-003 | Basix, assorted color |
| 15 mL Falcon Tubes | Becton Dickinson Labware Europe | 352009 | Polystyrene |
| 25 mL Serological Pipets | Fisher Scientific | 14-955-235 | |
| 70 μm cell strainer | Fisher Scientific | 08-771-2 | |
| Adult Brain Dissociation Kit | Miltenyi Biotec | 130-107-677 | Contains Enzyme P, Buffer Z, Buffer Y, Enzyme A, Buffer A, Debris Removal Solution |
| BSA | Sigma-Aldrich | A7906-50G | |
| Falcon 50 mL Conical Centrifuge Tubes | Fisher Scientific | 14-432-22 | |
| gentleMACS C Tubes | Miltenyi Biotec | 130-093-237 | |
| gentleMACS Octo Dissociator with Heaters | Miltenyi Biotec | 130-096-427 | |
| Gibco DPBS (1X) | ThermoFisher Scientific | 14190144 | |
| Glass Beaker | Fisher Scientific | 02-555-25A | |
| Pipette tips GP LTS 20 µL 960A/10 | Rainin | 30389270 | |
| Pipette Tips GP LTS 250 µL 960A/10 | Rainin | 30389277 | |
| Pipette tips RT LTS 1000 µL FL 768A/8 | Rainin | 30389213 | |
| Rainin Pipet-Lite XLS (2, 20, 200, 1000 μL) | Rainin | 30386597 | |
| Round Ice Bucket with Lid | Fisher Scientific | 07-210-129 | |
| Round-Bottom Tubes with Cell Strainer Cap | Falcon | 100-0087 |

