An In Vitro Transduction-Induced Myelination Assay for Oligodendrocyte Precursor Cells
Abstract
Source: Peckham, H. M., et al. Production and Use of Lentivirus to Selectively Transduce Primary Oligodendrocyte Precursor Cells for In Vitro Myelination Assays. J. Vis. Exp. (2015).
The video demonstrates a protocol for differentiating oligodendrocyte precursor cells (OPCs) into oligodendrocytes and inducing myelination in a co-culture with dorsal root ganglion (DRG) neurons. The OPCs are transduced with lentiviral vectors encoding a signaling protein that regulates myelination. The transduced OPCs are co-cultured with DRG neurons to assess the differentiation into oligodendrocytes and the signaling protein-induced myelination.
Protocol
1. Cloning of 2K7 Lentivector
- Before cloning the gene of interest into the 2K7 lentiviral vector, subclone the gene into the pENTR vector (3637 bp, Kanamycin resistant) using standard molecular techniques. Use the EcoRI and SacII restriction sites for the subcloning.
- Amplification of the 2K7 Lentivector
- Transform 2K7 lentivector DNA by gently mixing 100 ng of plasmid DNA with competent cells, and incubate on ice for 30 min.
- Heat shock DNA/ competent cells mix at 42 °C for 90 sec and plate them on LB-Agar plates containing both ampicillin (100 µg/ml) and chloramphenicol (15 µg/ml) at 37 °C for 16-18 hr.
NOTE: Chloramphenicol is used to prevent recombination between the long terminal repeats. - The next day, grow selected bacterial clones in Luria-Bertani (LB) media containing both ampicillin (100 µg/ml) and chloramphenicol (15 µg/ml) at 37 °C for 16-18 hr. Extract the DNA using a commercial plasmid DNA Maxiprep kit as per the manufacturer's instructions.
- Sub-cloning from pENTR vector to 2K7 Lentivector (Figure 1).
- Add the following to a 1.5 ml tube and mix gently: L4-R1 pENTR-pDNOR-CMV (promoter) (60 bng)1-2.5 µl L1-L2 pENTR4IRES2GFP (with gene of interest) (80 bng)1-2.5 µl K7 lentivector (80 ng/µl)1 µl TE-buffer, pH 82-5 µl.Total 8 µl.
- Remove the clonase enzyme mix from -80 °C and thaw on ice for 2 min. Ensure that this enzyme is a fresh aliquot from -80 °C as the enzyme has substantially reduced cloning efficiency with repeated freeze-thaw cycles
- Add 2 µl of enzyme per reaction and mix well via vortex, and incubate the clonase reaction mixture at 23-25 °C for 6-24 hr.
- Add 1 µl of proteinase K solution (supplied with enzyme kit) to the clonase reaction mixture and incubate for 10 min at 37 °C.
- Transform the clonase reaction mixture into competent cells and grow the select colonies in LB media containing ampicillin (100 µg/ml) at 37 °C for 16-18 hr.
- Extract and purify DNA using a commercial plasmid DNA Miniprep kit as per the manufacturer's instructions.
- Confirm the DNA via digestion using restriction enzymes Spe I and Sac II and an appropriate buffer as per the manufacturer's instruction to remove the fragment containing the promoter and gene of interest.
NOTE: This step is critical to check if the subcloned DNA has the correct insert gene of interest with the right vector backbone. The size of the vector itself without the inserted fragment is 6.7 kb. The size of the released inserted fragment includes both the promoter and the insert gene of interest. Calculate the expected fragment sizes from the digest for the specific gene via a DNA sequence editing program, e.g., APE-A Plasmid Editor. - Check the sizes of both the DNA vector backbone and the released fragment by running a 1% agarose gel in 1x TAE buffer (see Table 1) at 100 V.
- Amplify the confirmed DNA by growing bacteria in 500 ml of LB media containing ampicillin (100 µg/ml) at 37 °C for 16-18 hr.
- Extract and purify DNA using a commercial plasmid DNA Maxiprep kit as per the manufacturer's instructions.
NOTE: Maxiprep typically generates a sufficient amount of DNA required for viral production.
- Repeat steps 1.3.9-1.3.10 to amplify the following DNAs for lentiviral preparations: Envelope vector (PMD2.G, 6.1 kb), Package vector (pBR8.91, 12.5 kb), and an empty lentiviral vector as a control (GFP-CMV-2K7, 8.7 kb).
2. 2K7 Virus Production
NOTE: Day 1:
- On the day of transfection, plate 32 million HEK293 T cells in a T175 flask containing 25 ml Human Embryonic Kidney (HEK) 293 T cell media (see Table 1). Alternatively, plate 16 million cells the day before the transfection if time runs tight on the day of transfection.
NOTE: Transfection can be equally successful by plating cells on the day of transfection or prior to the day. Using either of the two alternatives, the critical point here is to make sure cells are stuck down on the culture dish surface prior to transfection.
NOTE: Day 2: - Transfection
- Prior to transfection, dilute DNA to 1 µg/µl in Tris-ethylenediaminetetraacetic acid (TE) Buffer that contains 10 mM Tris pH 8, 1 mM EDTA pH 8 in deionized water.
- In a 50 ml tube, prepare a master mix (Table 2) for transfection in the T175 flask. Add DNA to pre-warmed Dulbecco's Modified Eagle Medium (DMEM) and mix well by vortex, then add sterile polyethyleneimine (PEI) (see Table 1 to avoid premature precipitation.
- Mix well by inverting the tube 3-4x, and incubate for 15 min at room temperature (RT).
- Perform a full media change on HEK293T cells. Aspirate off the culture media from cells completely and feed with pre-warmed HEK293T cell culture media (25 ml per T175 flask).
- Add the transfection mixture (DNA/PEI mixture) dropwise to the monolayer cells. Move gently to mix well and incubate transfected cells at 37 °C, 5% CO2, overnight.
NOTE: Day 3: - To ensure the transfection is successful, check green fluorescence protein (GFP) expression 24 hours post-transfection via fluorescent microscopy.
NOTE: Over 50% of cells expressing GFP typically indicate a good transfection.
NOTE: Day 4:
- At 48 hr post-transfection, collect the viral supernatant and replace it with fresh HEK293 T media (25 ml per T175 flask).
- Centrifuge the viral supernatant at 1,140 x g for 10 min at 4 °C to clear up cell debris from the supernatant. Transfer the cleared supernatant to a 50 ml tube and store it at 4 °C.
NOTE: Day 5:
- Centrifuge the viral supernatant at 1,140 x g for 10 min at 4 °C to clear up cell debris from the supernatant. Transfer the cleared supernatant to a 50 ml tube and store it at 4 °C.
- At 72 hours post-transfection, collect the second batch of viral supernatant. Repeat step 2.3.1 and pool 48 and 72 hr cleared supernatants.
- To concentrate the virus, centrifuge viral supernatant at 170,000 x g for 90 min at 4 °C using 30 ml ultracentrifuge tubes.
- Discard the supernatant and repeat step 2.6 until all the cleared supernatant has been centrifuged leaving an (invisible) pellet of virus plus precipitated PEI in the base of the tube.
- To resuspend the virus, add 500 µl SATO media (see Table 1) to the ultracentrifuge tubes. Vortex for 30 sec and scrape the base of the tube with a pipette tip to mechanically loosen the virus. Repeat this step 6x in order to lose the viral pellet.
- Pool the resuspended virus into microcentrifuge tubes and spin very briefly to remove insoluble PEI. Filter the supernatant through a 0.45 µm filter to remove proteins.
- Aliquot the virus into 20 µl, 50 µl, and 100 µl aliquots and store at -80 °C.
3. Transducing OPCs
- Culture primary OPCs in SATO media (10ml/10cm plate) with Ciliary neurotrophic factor (CNTF, 10 ng/ml), Platelet-derived growth factor (PDGF, 10 ng/ml), Neurotrophin 3 (NT3, 1 ng/ml), and forskolin (4.2 µg/ml) at 37 °C, 8% CO2 for 24 hr after dissection.
- Completely aspirate off the OPC culture media, and feed cells with freshly made SATO media (10 ml) with growth factors (see above in step 3.1).
- Add virus to the OPCs to the optimal concentration, culture OPCs for a further 48 hr.
4. OPC Seeding for Myelinating Co-cultures (Figure 2)
- To remove OPCs off the surface, first rinse OPC plates with 8 ml Earle's balanced salt solution (EBSS) twice, then incubate cells with 5 ml of warm 0.05% Trypsin-EDTA diluted 1:10 with EBSS at 37 °C for 2 min.
- Neutralize the trypsin with 30% FBS in EBSS (5 ml) and remove cells from the plate by pipetting. Transfer cell suspension to a 15 ml tube, and centrifuge at 180 x g for 15 min at RT.
- Aspirate off the supernatant and resuspend the cell pellet in 1 ml pre-warmed SATO media followed by cell count.
- Prior to the OPC seeding, completely aspirate media from the DRG culture plate. Gently seed 200,000 OPCs dropwise onto the DRG neurons as previously described.
NOTE: The total OPC seeding volume must be less than 200 µl per 22 mm coverslip. - Leave cells to settle without moving the plate for 10 min in the tissue culture hood, then gently top up with 1 ml pre-warmed SATO media per well.
- Replate the remaining sister OPCs with SATO media with growth factors (see step 6.1). Use these sister OPCs to verify the expression of the protein of interest.
- After 24 hr, replace the SATO media with the co-culture media (2 ml/well) containing SATO media (no factors) and neurobasal (v/v) with 1% B2Maintain the co-cultures for 14 days with media change every 2-3 days.
- Assess the co-cultures for myelin protein expression by western blot analysis and visualize the formation of myelinated axonal segments by double-immunostaining with antibodies against myelin basic protein markers and neuronal markers.
Representative Results
Table 1. Table of stock solutions.
| Name | Ingredients | Notes |
| TE Buffer pH 8 | 10 mM Tris pH 8 1 mM EDTA pH 8 |
Make up in deionized water. |
| Polyethylenimine (PEI) | 1 g/L | Make up in deionized water, filter-sterilize, and store stocks at -20 °C. |
| LB Medium | 20 g Tryptone 10 g Yeast extract 10 g NaCl |
Make up to 2 L with deionized water |
| 50% Glycerol stock | Glycerol | Make up with an equal volume of deionized water and autoclave |
| HEK 293 T cell medium | DMEM 10% Fetal Bovine Serum 1% Penicillin/streptomycin 1% L-glutamine |
|
| TNE lysis buffer | 10 mM Tris pH 8 150 mM NaCl 1 mM EDTA pH 8 1% NP40 |
Dissolve NP40 in a smaller volume of deionized water first as it will crystallize on contact with water. Make up to the final volume in deionized water |
| M1 | MEM 10% Fetal Bovine Serum 0.4% D-glucose 2 nM L-glutamate 1% Penicillin/streptomycin |
For use with rat DRG neurons |
| mM1 | Neurobasal medium 2% B27 (SM1) supplement 0.4% D-glucose 2 nM L-glutamate 1% Penicillin/streptomycin |
For use with mouse DRG neurons |
| M2 | DMEM 10 mg/L Transferrin 5 mg/L Insulin 20 nM Progesterone 100 μM Putrescine 10 μM FdU 10 μM Uridine |
Make up M2 in a larger volume DMEM without FdU and uridine for use over 1-2 months. Add FdU and uridine to smaller volumes that can be finished within 2 weeks |
| mM2 | mM1 10 μM FdU 10 μM Uridine |
Add FdU and uridine to smaller volumes of medium that can be finished within 2 weeks. |
| 10x MT-PBS pH 7.4 | 28.48 g/L (160 mM) Na2HPO4·2H2O 5.52 g/L (40 mM) NaH2PO4·H2O 87.66 g/L (1.5 M) NaCl |
Add to deionized water and adjust pH to 7.4 with 10 N NaOH |
| 1x MT-PBS | 10x MT-PBS Deionized water |
Dilute 10x MT-PBS 1:10 in deionized water to make 1x MT-PBS |
| 10x Borate buffer (1.5 M) | 18.55 g Boric acid 1x MT-PBS |
Dissolve boric acid in 150 ml 1x MT-PBS. Adjust to pH 8.56 with 10 N NaOH and bring the final volume to 200 ml with 1x MT-PBS. Autoclave |
| 1x Borate buffer (0.15 M) | 10x Borate buffer 1x MT-PBS |
Prepare 100 ml of this buffer to dissolve PLN in. Add 10 ml of 10x borate buffer (pH 8.56) to 90 ml of 1x MT-PBS |
| 0.5 mg/ml Poly-L-ornithine (PLN) | 50 mg Poly-L-ornithine Hydrobromide 0.15 M Borate buffer (pH 8.56) |
Dissolve 50 mg of poly-L-ornithine hydrobromide in 100 ml of 1x borate buffer. Filter sterilize (0.22 μm filter) and store at 4 °C for up to one month |
| 100x Poly-D-lysine (PDL) | 5 mg Poly-D-lysine Sterile, deionized water |
100x PDL stocks can be frozen at -20 °C in single-use aliquots. Upon use, dilute to 1x in sterile, deionized water |
| Papain buffer | ||
| DNase | 12,500 U DNase I 1 ml EBSS |
On ice, dissolve the Dnase I in 1 ml of chilled EBSS. Aliquot (e.g., 300 μl/tube) and freeze overnight at -20 °C. Store at -20 °C. |
| 4% BSA | 8 g BSA 200 ml DPBS |
Dissolve the BSA in 150 ml DPBS at 37 °C. Adjust the pH to 7.4 with ~1 ml of 1 N NaOH. Bring the volume to 200 ml. Filter through a 0.22 μm filter to sterilize. Make 1 ml aliquots and store at -20 °C. |
| 10x Lo Ovomucoid | 3 g BSA 200 ml DPBS 3 g Trypsin inhibitor |
Add BSA to 150 ml DPBS and mix well. Add trypsin inhibitor and mix to dissolve. Add ~1 ml of 1 N NaOH to adjust the pH to 7. Bring the volume to 200 ml with DPBS. Filter-sterilize through a 0.22 μm filter. Make 1 ml aliquots and store at -20 °C. |
| 6x Hi Ovomucoid | 6 g BSA 200 ml DPBS 6 g trypsin inhibitor |
Add BSA to 150 ml DPBS and mix well. Add trypsin inhibitor and mix to dissolve. Add ~1 ml of 1N NaOH to adjust the pH to 7.4. Bring the volume to 200 ml with DPBS. Filter-sterilize through a 0.22 μm filter. Make 1 m aliquots and store at -20 °C. |
| SATO base | ||
| SATO media (rat) | 1% SATO base 1% Penicillin/streptomycin 1% 0.5 mg/ml Insulin 1% L-Glutamine 0.1% NAC 0.1% Biotin Make up with DMEM and filter sterilize. |
|
| SATO media (mouse) | 1% SATO base 1% 0.5 mg/ml insulin 1% Penicillin/streptomycin 1% L-Glutamine 0.1% NAC 0.1% Biotin 0.1% Trace Elements B 2% B27 Make up with DMEM and filter sterilize. |
|
| Insulin | 10 mg Insulin 20 ml Sterile deionzed water |
Add insulin to deionized water and add 100 μl of 1 N HCl to allow the insulin to dissolve. Mix well. Filter through a 0.22 μm filter and store at 4 °C for 4-6 weeks |
| NAC (N-Acetyl-L-cysteine) | 5 mg/ml NAC DMEM |
Dissolve NAC in DMEM to make a 5 mg/ml solution, aliquot, and store at -20 °C. |
| d-Biotin | 50 μg/ml biotin Sterile, deionized water |
Dissolve biotin in water to make a 50 μg/ml solution, aliquot, and store at -20 °C. |
| CNTF (ciliary neurotrophic factor) | 10 μg/ml CNTF 0.2% BSA in DPBS |
Dilute CNTF to make a 10 μg/ml solution with sterile 0.2% BSA in DPBS. Aliquot, flash freeze in liquid nitrogen and store at -80 °C. |
| PDGF (platelet-derived growth factor) | 10 µg/ml PDGF 0.2% BSA in DPBS |
Dilute PDGF master stock (prepared according to manufacturer's instructions) to 10 µg/ml with sterile 0.2% BSA in DPBS. Aliquot, flash freeze in liquid nitrogen and store at -80 °C. |
| NT3 (Neurotrophin-3) | 1 µg/ml NT-3 0.2% BSA in DPBS |
Dilute NT3 master stock (prepared according to manufacturer's instructions) to 1 µg/ml with sterile 0.2%BSA in DPBS. Aliquot, flash freeze in liquid nitrogen and store at -80 °C. |
| Forskolin | 50 mg Forskolin Sterile DMSO |
Add 1 ml sterile DMSO to the 50 mg bottle of forskolin and mix well to resuspend fully. Transfer to 15 ml tube and add 11 ml sterile DMSO to reach a concentration of 4.2 mg/ml. Aliquot and store at -20 °C. |
Table 2: Preparing transfection mix for 2K7 Virus.
| Vector | Concentration | Volume |
| pMDG.2 | 1 µg/µl | 5 µl |
| pBR8.91 | 1 µg/µl | 15 µl |
| 2K7 vector with GFP + gene of interest | 1 µg/µl | 22 µl |
| Sterile Polyethylenimine (PEI) | 1 g/L | 500 µl |
| DMEM | 2100 µl |
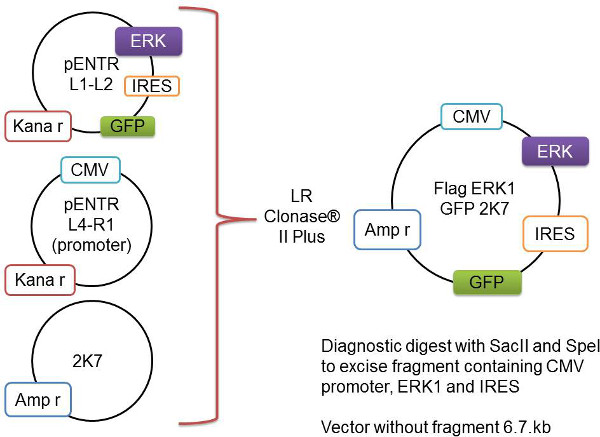
Figure 1: Schematic representation of the Gateway recombination process. The gene of interest, here represented as Flag-Erk1, is cloned into the pENTR L1-L2 vector. This is added to the pENTR L4-R1 vector containing the CMV promoter and the backbone 2K7 vector. These three vectors are recombined by the LR Clonase II Plus enzyme to insert the gene-of-interest and the promoter into the virus-ready 2K7 vector.
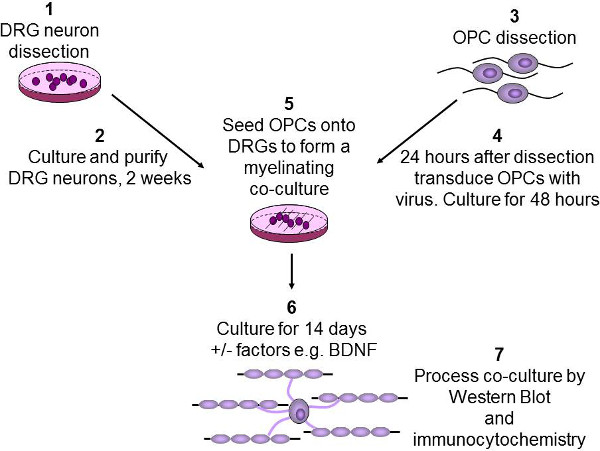
Figure 2: Schematic diagram of the in vitro myelination assay. DRG neurons are dissected from P2-3 rat pups, then purified and cultured over two weeks (1-2). OPCs are purified from P7-9 rat brains using immunopanning (3). OPCs are then infected with lentivirus and cultured for 48 hr (4). OPCs are then seeded onto DRGs, and any growth factors of interest such as BDNF are added (5). Co-cultures are then cultured for 2 weeks to allow OPCs to differentiate and myelinate the axons (6). Finally, co-cultures are either lysed for western blotting or fixed for immunocytochemistry (7).
Disclosures
The authors have nothing to disclose.
Materials
| 2K7 lentivector | Kind gift from Dr Suter | ||
| 5-Fluoro-2′-deoxyuridine | Sigma-Aldrich | F0503-100mg | |
| Ampicillin | Sigma-Aldrich | A9518-5G | |
| B27 – NeuroCul SM1 Neuronal Supplement | Stem Cell Technologies | 5711 | |
| BDNF (Human) | Peprotech | PT450021000 | |
| Biotin (d-Biotin) | Sigma-Aldrich | B4639 | |
| BSA | Sigma-Aldrich | A4161 | |
| Chloramphenicol | Sigma-Aldrich | C0378-100G | |
| CNTF | Peprotech | 450-13020 | |
| DAKO fluoresence mounting media | DAKO | S302380-2 | |
| DMEM, high glucose, pyruvate, no glutamine | Life Technologies | 10313039 | |
| DNase | Sigma-Aldrich | D5025-375KU | |
| DPBS | Life Technologies | 14190250 | |
| DPBS, calcium, magnesium | Life Technologies | 14040182 | |
| EBSS | Life Technologies | 14155063 | |
| EcoRI-HF | NEB | R3101 | |
| Entry vectors for promoter and gene of interest | Generate as per protocols 1-2 | ||
| Fetal Bovine Serum | Sigma-Aldrich | 12003C | |
| Forskolin | Sigma-Aldrich | F6886-50MG | |
| Glucose (D-glucose) | Sigma-Aldrich | G7528 | |
| Glycerol | Chem Supply | GL010-500M | See stock solutions |

