Differentiation of Neural Stem Cells to Neurons Using a Compartmentalized Microfluidic Device
Abstract
Source: Paranjape, S. R., et al. Compartmentalization of Human Stem Cell-Derived Neurons within Pre-Assembled Plastic Microfluidic Chips. J. Vis. Exp. (2019)
The video demonstrates the differentiation of neural stem cells (NSCs) using a microfluidic device comprising a somatic and an axonal compartment separated by a microgroove barrier. NSCs are introduced into the somatic compartment and are allowed to adhere. The NSCs differentiate into neurons inside the somatic compartment and extend axons through the microgroove barrier to the axonal compartment.
Protocol
NOTE: Figure 1A, B shows a schematic of the pre-assembled cyclic olefin copolymer (COC) chip, including the locations of the main channels or compartments, wells, and microgrooves. The compartmentalized chip can establish distinct fluidic microenvironments within a compartment as demonstrated by the isolation of food coloring dye (Figure 1C).
1. Coating of multi-compartment chips for hSC-neurons
NOTE: Figure 2A shows an overview of the coating procedures.
- Dissolve poly-L-ornithine in cell culture-grade distilled water to make 600 µL of solution per chip of a 20 µg/mL working solution.
- Aspirate the remaining phosphate-buffered saline (PBS) left after preparing the chips from the wells. When aspirating, ensure that the pipet tip is away from the channel opening.
NOTE: The microfluidic channels must remain filled for the entirety of this procedure. Do not aspirate liquid from the channels/compartments, only the wells. - Load 150 µL of poly-L-ornithine working solution in the upper right well. Wait for 90 seconds or until the liquid begins to fill the lower right well. Add 150 µL of media to the lower right well and wait for 5 min to let the liquid pass through the microgrooves.
- Add 150 µL of media to the upper left well. Wait 90 s and then add 150 µL to the lower left well. Wrap the holder of the chip(s) with parafilm and place at 4 °C overnight.
NOTE: Alternatively, place the chip and holder at 37 °C for 1 h. - Aspirate the media from the wells, ensuring that the pipet tip is placed away from the channel opening. Add 150 µL of sterile water to the upper right well. Wait for 90 s and then add 150 µL to the lower right well. Wait for 5 min to let the liquid pass through the microgrooves.
- Add 150 µL of sterile water to the upper left well, wait for 90 s, and then add another 150 µL to the lower left well.
- Repeat steps 1.5-1.6.
- Thaw laminin slowly at 2 °C to 8 °C and prepare a 10 µg/mL working solution.
- Load 150 µL of the laminin working solution to the upper right well. Wait for 90 seconds or until the liquid begins to fill the lower right well. Pipet 150 µL to the lower right well. Wait for 5 min to let the laminin go through the microgrooves.
- Add 150 µL of laminin to the upper left well. Wait 90 s and then add 150 µL to the lower left well. Incubate the chip within its holder for 2 h at 37 °C.
- Rinse the chip with PBS (without Ca2+ and Mg2+). Load 150 µL of PBS to the upper right well. Wait for 90 seconds or until the liquid begins to fill the lower right well. Add 150 µL of PBS to the lower right well and wait for 5 min to let the PBS go through the microgrooves.
- Pipet 150 µL of PBS to the upper left well. After 90 s pipet 150 µL to the lower left well.
- Aspirate PBS from the chip and then rinse the chip with NSC media (see Table of Materials). Load 150 µL of media to the upper right well. After 90 s or when liquid passes through the right channel into the lower right well, add 150 µL to the lower right well. Wait for 5 min to let the media flow through the microgrooves.
- Add 150 µL of media to the upper left well and another 150 µL to the lower left well. Incubate the chip in a 37 °C incubator with 5% CO2 to pre-condition the chip until ready to seed NSCs.
NOTE: Chips can be pre-conditioned overnight if needed.
2. Seeding NSCs into the multicompartment chip
NOTE: This protocol used commercially purchased NSCs, unlike our previous publication which began with human embryonic stem cells. In this protocol neural stem cells are plated directly into the plastic chips where they differentiate into neurons. The cells can be allowed to proliferate as NSCs (without differentiation) for up to 2 passages and stored in vials in liquid N2 for further use. This protocol uses NSC vials stored in liquid N2 after passage 1 or 2. Figure 2B shows an overview of the coating procedures.
- Thaw NSCs according to the manufacturer's instructions.
NOTE: This procedure applies to H9-derived NSCs. Other cell lines will require cell density optimization. - Count the cell concentration using a hemocytometer and adjust using NSC media to get a concentration of 7 x 106 cells per mL.
- Aspirate media from each well of the chip. Avoid aspirating media from the main channels.
- Pipet 5 µL of the cell solution to the upper right well, followed by 5 µL to the lower right well (70,000 cells total). Ensure that the cells are pipetted towards the main channel openings. Use a microscope to check that cells have entered the channel. Wait 5 min for cells to adhere to the bottom of the chip.
NOTE: Either or both compartments can be used to load cells. - Pipet ~150 µL of NSC media to the upper right well and then 150 µL to the lower right well. Repeat on the left side. Incubate at 5% CO2, 37 °C within a suitable humidified container.
- After 48 hours, aspirate NSC media from the wells and replace it with neural differentiation media by adding 150 µL to each top well and each bottom well.
- Culture neurons within a 5% CO2, 37 °C incubator within a suitable humidified container.
Representative Results
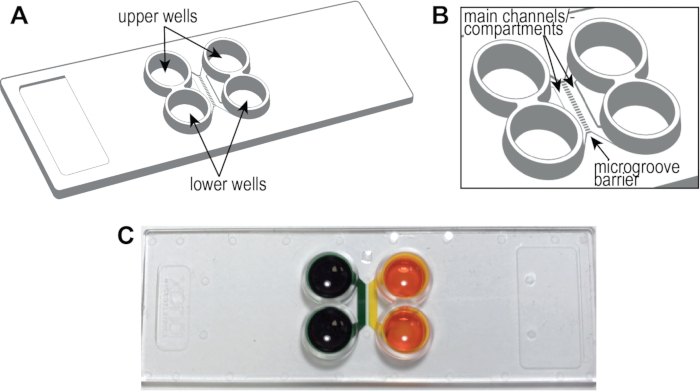
Figure 1: The pre-assembled COC, multicompartment microfluidic chip. (A) A drawing of the chip identifying the upper and lower wells. The size of the chip is 75 mm x 25 mm, the size of standard microscope slides. (B) A zoomed-in region showing the channels and microgrooves separating the channels. (C) This photograph illustrates the creation of isolated microenvironments within each compartment using food coloring dye.
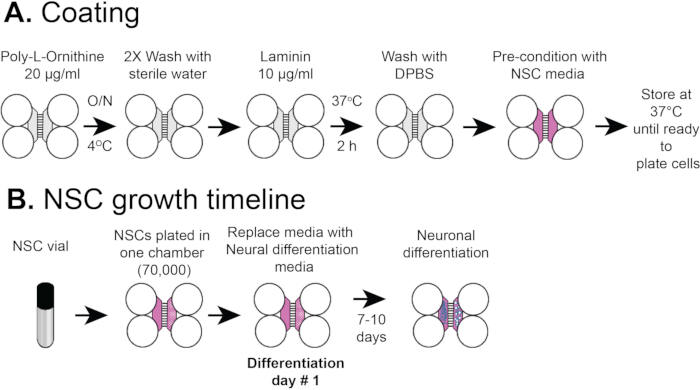
Figure 2: Plastic compartmentalized chip coating and NSC cell plating timeline. (A) Plastic multicompartment chips were coated with a pre-coating solution, and then poly-L-ornithine and laminin before pre-conditioning with NSC media. (B) NSCs were plated with 7 x 104 cells in the somatic compartment of the chip. The cells were grown in NSC media for 24 h and then the media was replaced with neural differentiation media. Differentiated neurons were observed by 7-10 days post differentiation.
Disclosures
The authors have nothing to disclose.
Materials
| complete neural stem cell media: | |||
| REC HU EGF 10 UG BIOSOURCE (TM) |
ThermoFisher Scientific | PHG0314 | 20ng/mL |
| REC HU FGF BASIC 10 UG BIOSOURCE (TM) |
ThermoFisher Scientific | PHG0024 | 20ng/mL |
| GlutaMAX Supplement (100X) | ThermoFisher Scientific | 35050061 | 2mM |
| KnockOut DMEM/F-12 | ThermoFisher Scientific | 12660012 | |
| StemPro Neural Supplement | ThermoFisher Scientific | A1050801 | 2% |
| Gibco DPBS without Calcium and Magnesium | ThermoFisher Scientific | 14190144 | |
| GIBCO HUMAN NSC (H9) KIT COMBO KIT | Gibco | N7800200 | |
| Gibco Laminin | ThermoFisher Scientific | 23017015 | |
| Glass Pasteur pipettes | Sigma-Aldrich | CLS7095D5X SIGMA | 5.75 in length |
| H9-DERIVED HU NEURAL STEM CELL 1E6 CELLS/VIAL; 1 ML |
ThermoFisher Scientific | 510088 | |
| hibernate-E Medium | ThermoFisher Scientific | A1247601 | |
| Incubator, 5% CO2 37 °C | |||
| Mr. Frosty | ThermoFisher Scientific | 5100-0001 | |
| Neural differentiation media | Per 100 mL. | ||
| Antibiotic-Antimycotic (100x) | ThermoFisher Scientific | 15240112 | 1mL (100X) |
| Ascorbic acid | Sigma Aldrich | A8960 | 200mM |
| BDNF | ThermoFisher Scientific | PHC7074 | 40 ng/mL |
| Gibco B27 Plus Supplement (50X) | FisherScientific | A3582801 | 2mL (50X) |
| Gibco CultureOne Supplement (100X) | FisherScientific | A3320201 | 1mL (100X) |
| Gibco Neurobasal Plus Medium | FisherScientific | A3582901 | |
| StemPro Accutase Cell Dissociation Reagent | ThermoFisher Scientific | A1110501 | |
| XC pre-coat | Xona Microfluidics, LLC | XC Pre-Coat | included with XonaChips |
| XonaChip | Xona Microfluidics, LLC | XC450 | 450 µm length microgroove barrier |
| Humidifier Tray | Xona Microfluidics, LLC | humidifier tray |

