Culturing Murine Spiral Ganglion Neuron Explants on Multielectrode Arrays
Abstract
Source: Hahnewald, S., et al Spiral Ganglion Neuron Explant Culture and Electrophysiology on Multi Electrode Arrays. J. Vis. Exp. (2016)
This video demonstrates the process for isolating spiral ganglion explants from the inner ear and co-culturing them with the organ of Corti on a multielectrode array. Spiral ganglia are carefully extracted and sectioned into smaller tissue sections or explants. These explants are cultured on the multielectrode array's active area to promote neuronal process outgrowth.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Prepare Solutions for Experiments
- Prepare the extracellular matrix (ECM) coating solution (see Material Table): Thaw ECM mix on ice. Dilute the ECM mix 1:10 in basic culture medium (without neurotrophic factors or Fetal Bovine Serum (FBS)) and store on ice.
- Prepare the culture medium (see Material Table): Prepare a stock of Neurobasal medium not containing FBS or Brain-Derived Neurotrophic Factor (BDNF). Supplement neurobasal media with fresh FBS (10%) and BDNF (5 ng/ml) just before adding to the cell culture.
- Prepare the extracellular solution (see Material Table).
2. Washing and Sterilization of MEAs
NOTE: The MEAs used for the experiments contain 68 electrodes arranged in a rectangular grid (Figure 1E). Each electrode has a size of 40 x 40 µm2 with a spacing of 200 µm from center to center. The electrodes are made of platinum. The electrodes are connected to the corresponding contacts by a circuit made of indium tin oxide. This circuit is insulated by a 5 μm layer of SU-8. See Material table for details on the provider. Other MEA layouts may be suitable for these experiments.
- For new MEAs: Rinse them by immersing in 70% ethanol for 30 sec and wash with distilled water for 30 sec. Work in a laminar flow hood.
- Let the MEA dry for 30 min in a laminar flow hood.
- Put each MEA into an individual sterile Petri dish (35 mm x 10 mm) and seal with foil. The MEAs can be stored until used.
- For used MEAs: Incubate the MEAs in a Petri dish (35 mm x 10 mm) containing 1 ml of enzymatic solution (see Materials Table) O/N on orbital shaker at RT to remove biological material. Then continue as in step 2.1.
NOTE: Handle the MEAs with care using forceps with rubber-covered tips.
3. Preparation of MEAs for Culture Experiments
- Remove the sealing foil and leave the MEA in the Petri dish (35 mm x 10 mm) for the whole experiment.
- Coat the MEAs with the solution prepared in 1.1: Use a cold 200 µl pipette tip (stored at -20 °C) to pipet 50 µl of coating solution to each MEA, covering the whole electrode area.
- Allow MEAs to coat for 30 min to 1 hr at RT.
- Remove the coating solution using a pipette. Apply 100 µl of culture medium supplemented with 10% FBS and 5ng/ml BDNF and leave it at RT until plating the tissue.
- Place 2 Petri dishes containing the coated MEAs into a large petri dish (94 mm x 16 mm) and add a third small petri dish containing 1.5 ml of Phosphate Buffered Saline (PBS) for humidification.
NOTE: Adding a small petri dish (step 3.5) containing PBS is crucial to significantly minimize evaporation of culture medium.
4. Spiral Ganglion Dissection
NOTE: Gross dissection can be done outside the laminar flow hood (Step 4.1 to 4.4). For fine dissection sterile conditions (laminar flow hood) are mandatory (from step 4.5).
- Euthanize animals (5-7 day old mice) by decapitation without prior anesthesia.
- Sterilize the head by spraying with 70% ethanol.
- By holding the head, cut the connection between the skin and the skull with a sharp/sharp scissor along the sagittal line.
- Cut the skull sagittally and remove the brain using sharp/blunt scissors.
- Cut the temporal bones from the skull and place those in a petri dish containing sterile ice cold Hanks' Balanced Salt Solution (HBSS).
- Using a dissection microscope, dissect the tympanic bulla using fine forceps and isolate the inner ear.
- Remove the bone of the cochlea, using fine forceps.
- Remove the spiral ligament and stria vascularis (SV) together by holding with forceps the basal portion of the spiral ganglion (SG) and the SV and slowly unwinding the SV from base-to-apex
- Isolate the organ of Corti (OC), the SG and the modiolus (Figure 1A–C).
- Separate the OC from the SG and modiolus by holding with forceps the basal portion of the SG and the OC and slowly unwinding the OC from base-to-apex.
- Cut lateral explants (200 to 500 µm in diameter) from the spiral ganglion still attached to the modiolus (Figure 1D) using forceps and a micro scalpel.
5. Spiral Ganglion Explant Culture on MEAs
- Place two spiral ganglion explants next to the electrodes on the MEA previously prepared with 100 µl of culture medium.
- Place the organ of Corti approximately 5 mm away from the electrode area.
- Use forceps to pin the explants and the organ of Corti onto the MEA while avoiding damaging the tissue.
- Place the MEAs carefully into the incubator and culture at 37 °C and 5% CO2. The next day, visually inspect that the explants have attached to the MEA.
NOTE: If explants have not attached O/N, they will rarely do so over the next few days. The OC is placed adjacent to the culture for trophic/neurotrophic support. - Add 100 µl of culture medium containing 10% FBS and BDNF daily for 5 consecutive days.
- On day 6, add 2 ml of culture medium containing 10% FBS and 5ng/ml BDNF and culture the tissue for an additional 13 days.
Representative Results
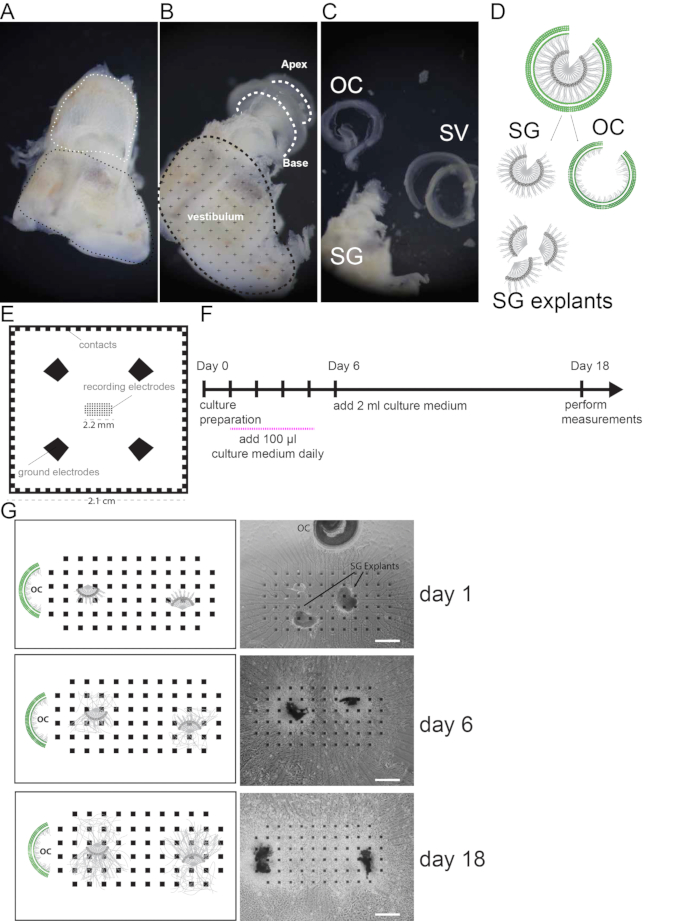
Figure 1. Cell culture preparation. (A) Freshly dissected mouse inner ear. White dashed line indicates the location of the cochlea, black dashed line indicates the vestibular region. (B) Mouse inner ear after removal of the cochlear bony wall. Cochlear turns are indicated by white dashed lines. (C) The Spiral ganglion (SG) and modiolus, the organ of Corti (OC) and the stria vascularis (SV) and spiral Ligament are shown after dissection. (D) Schematic of SG and OC dissection and SG explant preparation. (E) Illustration of the multi electrode array used in this study. Recoding electrodes are organized in a rectangular grid in the center and occupy an area of 2.2 mm2. 4 Ground electrodes and side contacts are illustrated. (F) Schematic of the culture protocol. Recordings are performed at day 18. (G) Representative pictures (bright field images) and schemes of SG explants on MEA monitored in culture at day 1, 6 and 18. Scale bars = 400 µm.
Disclosures
The authors have nothing to disclose.
Materials
| Neurobasal medium | Invitrogen | 21103-049 | 24 ml (for 25 ml) |
| HEPES | Invitrogen | 15630-080 | 250 μl (for 25 ml) |
| Glutamax | Invitrogen | 35050-061 | 250 μl (for 25 ml) |
| B27 | Invitrogen | 17504-044 | 500 μl (for 25 ml) |
| FBS | GIBCO | 10099-141 | 10% (for 25 ml) |
| BDNF | R&D Systems | 248-BD-025/CF | Final 5 ng/ml (for 25 ml) |
| NaCl | 145mM | ||
| KCl | 4mM | ||
| MgCl2 | 1mM | ||
| CaCl2 | 2mM | ||
| HEPES | 5mM | ||
| Na-pyruvate | 2mM | ||
| Glucose | 5mM | ||
| PBS | Invitrogen | 10010023 | |
| BSA | Sigma | A4503-50G | 2% |
| Triton X-100 | Sigma | X100 | 0.01% |
| Petri dish 35 mm | Huberlab | 7.627 102 | |
| Petri dish 94 mm | Huberlab | 7.633 180 | |
| Dumont #5 tweezer | WPI | 14098 | |
| Dumont #55 tweezer | WPI | 14099 | |
| Enzymatic solution: Terg-a-Zyme | Sigma | Z273287-11KG | |
| Extracellular Matrix (ECM) mix: Matrigel TM | Corning | 356230 | |
| MEA electrodes | Qwane Biosciences | (Lausanne, Switzerland) |

