Performing Injection into Orbital Lobe of Superior Lacrimal Gland: A Technique for Inducing Dry Eye Disease in Rabbit Model
Abstract
Source: Honkanen, R. A. et al, A Rabbit Model of Aqueous-Deficient Dry Eye Disease Induced by Concanavalin A Injection into the Lacrimal Glands: Application to Drug Efficacy Studies. J. Vis. Exp. (2020).
This video describes the procedure to induce dry eye disease in a rabbit model through an injection of Concanavalin A into the orbital lobe of the superior lacrimal gland. The developed model is useful to study the effectiveness of various drugs for treating dry eye disease.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Animal preparation
NOTE: The portions of the orbital lacrimal gland system are injected.
- Sedate the rabbits with acepromazine 0.2 mg/kg subcutaneously.
- Shear off the fur in the periorbital and scalp area and completely remove any residual fur using Nair. Leave the skin entirely smooth for better visualization of the anatomical hallmarks and US-guided injection of concanavalin A (Figure 1).
- Induce moderate sedation as described above.
2. Injection of the orbital superior lacrimal gland (OSLG)
NOTE: OSLG follows in rapid succession.
- Apply medial pressure to the globe causing the OSLG to protrude from the posterior incisure. Apply medial pressure to the globe (Figure 2, red arrow) with the protuberance of the OSLG from the posterior incisure. The protuberance serves as gross localization to find the posterior incisure.
- Use curved forceps with tips closed to indent the area until the bony opening in the skull is felt. This will be slit-like with an anterior/posterior direction under the protuberance.
- Apply modest pressure with forceps to leave an indentation in the skin, which will serve as the landmark for needle placement (Figure 3A).
- Insert a needle (tuberculin syringe with a 27-gauge, 5/8-inch needle) perpendicular to the skin over the indentation mark (Figure 3B) ~ ¼ inch into the incisure, then redirect the needle posteriorly and externally towards the lateral canthus aiming for the midpoint between the injection site and bony orbital rim.
NOTE: If the incisure is not precisely targeted with the needle, the skull blocks its advancement. - Once the hub of the needle is reached, slowly inject 1000 µg of Con A in a volume of 0.2 mL (Figure 3C).
- Complete the injection of both the PSLG and OSLG within 2-3 min
- Remove the animal from isoflurane sedation (if not yet done). Injection of the inferior lacrimal gland (ILG) can usually be completed without further sedation.
Representative Results
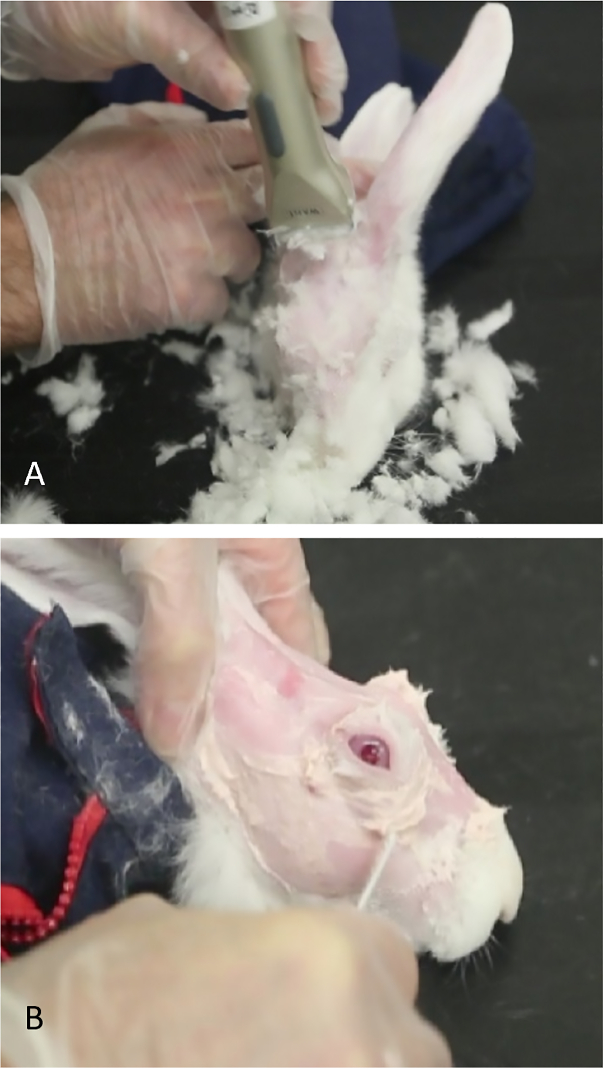
Figure 1: Preparation of rabbit for concanavalin A injections. (A) Small shears are used to remove fur, allowing easier visualization of landmarks to identify the orbital superior lacrimal gland. (B) Nair is used to remove hair that remains after shearing.
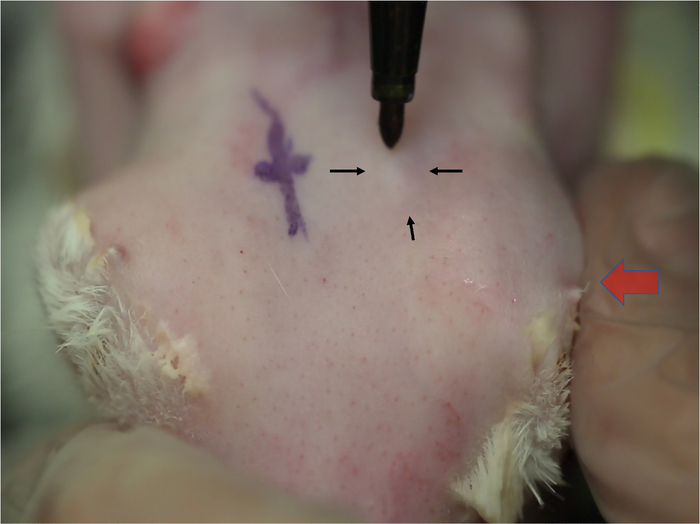
Figure 2: Localization of the orbital superior lacrimal gland. Changes in skin contours indicate the location of the OSLG as it protrudes through the posterior incisure. Alternating medial pressure on the globe (large arrow) causes the superior orbital gland to prolapse, which is seen as a small elevation in the skin. This elevation will increase in size each time the pressure is applied (small arrows). The location of this gland is usually in line with the posterior orbital rim.
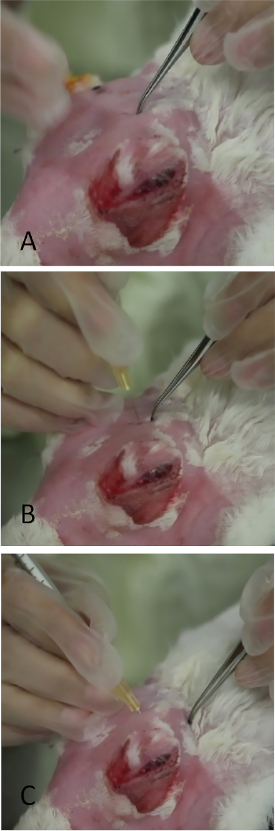
Figure 3: Injection of the orbital superior lacrimal gland. (A) Application of gentle pressure to the skull with fine-toothed forceps in the area which prolapsed as in Figure 2. A thin slit-like opening in the skull can be palpated. Leaving a small indentation mark with the forceps greatly aids placement of the needle during injection. (B) The needle is being inserted perpendicularly through the incisure. If placed incorrectly, its passage is stopped by the bony skull. (C) The needle is in final position angled towards the lateral canthus.
Disclosures
The authors have nothing to disclose.
Materials
| 100 mm macro lens | Canon EF 100mm f/2.8L IS USM | 3554B002 | |
| 26 gauge needles (5/8) | Becton Dickinson and Company, Franklin Lakes, NJ |
305115 | Needles for injecting ConA into the lacrimal glands |
| 27 gauge needles (5/8) | Becton Dickinson and Company, Franklin Lakes, NJ |
305921 | Needles for injecting ConA into the lacrimal glands |
| Aceproinj (acepromazine) | Henry Schein Animal Health, Dublin, OH |
NDC11695-0079-8 | 0.1ml/kg subcutaneously injection for rabbit sedation |
| Anesthesia vaporizer | VetEquip, Pleasanton, CA | Item #911103 | |
| Bishop Harmon Forceps | Bausch and Lomb (Storz), Bridgewater, NJ |
E1500-C | Tissue forceps |
| Caliper | Bausch and Lomb (Storz), Bridgewater, NJ |
E-2404 | Caliper used to measure length of needle during ConA injection |
| Concanavalin A | Sigma, St. Louis, MO | C2010 | Make 5mg/ml in PBS for injection into rabbit lacrimal glands |
| Rabbit, New Zealand White or Dutch Belted (as described in text) |
Charles River Labs, Waltham, MA | 2-3 kg | Research animals |
| Surgical Loupes +1.50 | Designs for Vision, Bohemia, NY | Specialty item | Provide magnificantion of ocular surface while observing tear break up and performing Concanavalin A injections. |

