Cerebral Blood Oxygenation Measurement Based on Oxygen-dependent Quenching of Phosphorescence
Summary
We present an experimental procedure for measuring the partial pressure of oxygen (pO2) in cerebral vasculature based on oxygen-dependent quenching of phosphorescence. Animal preparation and imaging procedures were outlined for both large field of view CCD-based imaging of pO2 in rats and 2-photon excitation based imaging of pO2 in mice.
Abstract
Monitoring of the spatiotemporal characteristics of cerebral blood and tissue oxygenation is crucial for better understanding of the neuro-metabolic-vascular relationship. Development of new pO2 measurement modalities with simultaneous monitoring of pO2 in larger fields of view with higher spatial and/or temporal resolution will enable greater insight into the functioning of the normal brain and will also have significant impact on diagnosis and treatment of neurovascular diseases such as stroke, Alzheimer’s disease, and head injury.
Optical imaging modalities have shown a great potential to provide high spatiotemporal resolution and quantitative imaging of pO2 based on hemoglobin absorption in visible and near infrared range of optical spectrum. However, multispectral measurement of cerebral blood oxygenation relies on photon migration through the highly scattering brain tissue. Estimation and modeling of tissue optical parameters, which may undergo dynamic changes during the experiment, is typically required for accurate estimation of blood oxygenation. On the other hand, estimation of the partial pressure of oxygen (pO2) based on oxygen-dependent quenching of phosphorescence should not be significantly affected by the changes in the optical parameters of the tissue and provides an absolute measure of pO2. Experimental systems that utilize oxygen-sensitive dyes have been demonstrated in in vivo studies of the perfused tissue as well as for monitoring the oxygen content in tissue cultures, showing that phosphorescence quenching is a potent technology capable of accurate oxygen imaging in the physiological pO2 range.
Here we demonstrate with two different imaging modalities how to perform measurement of pO2 in cortical vasculature based on phosphorescence lifetime imaging. In first demonstration we present wide field of view imaging of pO2 at the cortical surface of a rat. This imaging modality has relatively simple experimental setup based on a CCD camera and a pulsed green laser. An example of monitoring the cortical spreading depression based on phosphorescence lifetime of Oxyphor R3 dye was presented. In second demonstration we present a high resolution two-photon pO2 imaging in cortical micro vasculature of a mouse. The experimental setup includes a custom built 2-photon microscope with femtosecond laser, electro-optic modulator, and photon-counting photo multiplier tube. We present an example of imaging the pO2 heterogeneity in the cortical microvasculature including capillaries, using a novel PtP-C343 dye with enhanced 2-photon excitation cross section.
Click here to view the related article Synthesis and Calibration of Phosphorescent Nanoprobes for Oxygen Imaging in Biological Systems.
Protocol
1. Wide field of view imaging of pO2 in cortical vasculature of a rat
- Rats (250 g -350 g) are initially anesthetized with isoflurane, and the femoral artery and vein are catheterized to monitor heart rate, blood pressure, and blood gases, as well as for intravenous infusion. Body temperature is maintained at 37 ± 0.1 °C. Tracheotomy is performed, and rats are ventilated with a mixture of air and oxygen.
- Blood samples for measuring the blood gases are taken every 30-45 min and ventilation and anesthesia are adjusted to keep the blood gases within normal physiological range.
- A closed cranial window on the parietal bone 4 mm x 4 mm in size is prepared for imaging. The bone and dura are removed and the cranial window is filled with 1.5% agarose and sealed with a microscope coverslip. An additional 1 mm2 burr hole on the frontal bone is used to induce CSD by intracortical microinjection of KCl (~10 μl, 1 M). Extreme care should be taken to avoid any damage of the cortical vasculature. Excess heat, mechanical pressure, or brief period of hypoxia may compromise blood brain barrier and cause leakage of the dye from the vasculature into interstitial space.
- A mask from optically opaque material is placed around cranial window. The purpose of the mask is to absorb phosphorescence signal that is coming from the dye that leaked into the tissue from the edges of the dura matter. The dye in the interstitial space is the source of the very bright phosphorescence that can spoil the measurement. The increased brightness of the dye in interstitial space is result of build up of the dye in environment with low oxygen pressure and low quenching rate. The damage of the vasculature in experiment is therefore easily recognized by appearance of bright phosphorescence spots, in which case the experimental data is rejected.
- After completion of the surgery, isoflurane is discontinued and anesthesia is switched to alphachloralose (50 mg/kg intravenous bolus followed by 40 mg/(kg h) infusion).
- Animal is transferred on a cart with ventilator, blood pressure and temperature monitor. While breathing the room air, the animal is quickly moved to the imaging setup and reattached to the flowmeter with appropriate mixture of air and oxygen.
- The stereotaxic frame that holds the animal is placed under the objective. The cranial window is centered in the field of view under the objective and positioned parallel to the focal plane.
- The pulsed laser is turned on and set to minimal pulse energy. The optical beam is directed toward pulse energy meter and pulse energy delivered to the sample is adjusted to be not more than 10 mJ/cm2. The beam iss centered on the cranial window with oblique incidence angle of ~60 degrees and laser iss turned off.
- Blood gas measurements and adjustments of the ventilation and anesthesia are performed until all physiological parameters of the animal were within normal physiological range.
- Assuming that blood volume is approximately 7% of the body weight, an appropriate amount of the phosphorescence probe Oxyphor R3 is dissolved in 1 mL of saline to achieve 4 x 10-5 M blood concentration of the probe. The probe solution is injected via the femoral vein.
- A 1 M solution of potassium chloride is prepared and ~1 ul is injected with the syringe through the burr hole on the frontal bone to induce CSD.
- The laser is turned on and imaging is started immediately after injection of the KCl solution. Imaging of phosphorescence is performed during ~10 min.
- Another blood gas measurement is performed to confirm that animal physiological parameters are still within normal range.
- Phosphorescence lifetimes are obtained for all pixels by fitting the single exponential decay using nonlinear square fitting with statistical weighting in Matlab. Phosphorescence lifetimes are converted to the pO2 values using an empirical Stern Volmer-like relationship (vide infra).
2. High resolution two-photon pO2 imaging in cortical micro vasculature of a mouse
- Mice are anesthetized with isofluorene and femoral artery is catheterized to monitor heart rate, blood pressure, and blood gases, as well as for administration of the dye. Body temperature is maintained at 37 ± 0.1 °C and animals are spontaneously breathing a mixture of air and oxygen.
- A cranial window is prepared according to technique originally developed by David Kleinfeld and Winfried Denk. A good care should be taken to avoid any damage of the vasculature to prevent possible leakage of the dye into interstitial space.
- A few blood samples for measuring the blood gases are taken during a whole preparation procedure and ventilation and anesthesia are adjusted to keep the blood gases within normal physiological range.
- The animal is moved to the imaging setup. A modified stereotaxic frame that holds the animal is placed under the objective. The cranial window is centered in the field of view under the Olympus 4X objective and positioned parallel to the focal plane.
- An image of the cranial window is taken with the digital camera through the eyepieces, and the 4X objective is replaced with the Olympus 20X objective (NA = 0.95).
- Blood gas measurements and adjustments of the ventilation and anesthesia are performed until all physiological parameters of the animal are within a normal physiological range.
- Assuming that blood volume is approximately 7% of the body weight, an appropriate amount of the phosphorescence probe PtP-C343 is dissolved in 0.2 mL of saline to achieve ~15 μM blood concentration of the probe. The probe solution is injected via the femoral artery.
- Begin the experiment by setting the desired time intervals for dye excitation and emission collection at each pixel.
- Acquire a survey scan which is a slow 2-dimensional raster scan of phosphorescence intensity decay that displays the vasculature structure at the current depth. The objective is moved perpendicular to the cranial window (Z axis) and slow 2D survey scans of phosphorescence intensity are taken using the minimal laser power at 840 nm to find the microvessels in the imaging plane.
- At each imaging depth, based on the survey scan of phosphorescence at that depth, a set of points within the vasculature are selected, and recording of phosphorescence decays at each point is repeated for a predefined number of averaging.
- Set the desired amount of averaging, measurement interval, and experiment duration and start the measurement. pO2 measurements are acquired at the selected locations at the specified measurement interval for the duration of the experiment.
- During measurements, the software re-directs the excitation laser to the selected locations by changing the position of the galvanometer scanning mirrors. Control of the galvanometer scanners, electro optical modulator, and all other equipment are performed by custom made software in LabView.
- Phosphorescence lifetimes are obtained for all selected points by fitting the single exponential decay using nonlinear square fitting in Matlab. Phosphorescence lifetimes are converted to the pO2 values using an empirical Stern Volmer-like relationship (vide infra).
- After collecting the data at various cortical depths, inject dextran-conjugated fluorescein dye into vasculature for imaging the microvasculature structure.
- Obtain a stack of structural images of the vasculature by performing the two-photon imaging of FITC fluorescence using a green channel in the four-channel detector.
- Experiments on animals were performed in accordance with the guidelines and regulations set forth by Massachusetts General Hospital Subcommittee on Research Animal Care.
3. Representative Results:

Figure 1. Panel (a) on the left side of this figure displays a wide field of view image of oxygen pressure before the arrival of a CSD wave. Panel (b) on the right side shows the temporal evolution of the average oxygen pressure during CSD propagation within the region of interest marked on panel (a).
Figure 2 (avi movie): This movie shows the temporal evolution of the oxygen pressure in the whole cranial window during propagation of the CSD wave. Scale bar indicates oxygen pressure in millimeters of mercury.
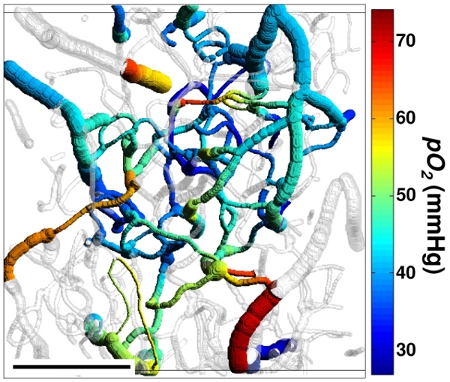
Figure 3. 3D projection of the imaged vasculature stack. The shades of grey represent a volumetric vessel mask, created based on the structural image.
Measured pO2 values are color-coded. Scale bar is 200 micrometers. Reprinted with permission from Nature Publishing Group.7
Discussion
We demonstrated two applications of the pO2 measurement in cortical microvasculature based on oxygen-dependent quenching of phosphorescence. While the first method based on CCD imaging provides wide field of view monitoring of pO2, measuring partial pressure of oxygen in cortical microvasculature based on 2-photon microscopy provides capillary resolution and allows imaging in depth. Both methods provide high speed and high signal-to-noise measurements. In addition, the phosphorescence lifetime measurement of pO2 is largely insensitive to the changes in the optical parameters of the tissue during the experiment, which is usually a concern for the other optical imaging techniques that have a contrast mechanism based on intensity. Presented instruments allow quantitative analysis of the dynamic delivery of oxygen and the brain tissue metabolism that will lead to a better understanding of neurovascular coupling in normal and diseased brain
Acknowledgements
We would like to acknowledge support from US National Institutes of Health grants R01NS057476, P50NS010828, P01NS055104, R01EB000790, K99NS067050, R01HL081273 and R01EB007279 and American Heart Association grant 0855772D.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Glycopyrrolate | Reagent | American Regent Inc. | NDC 0517-4605-25 | Used to control pharyngeal, tracheal, and bronchial secretions. |
| Lidocaine HCL | Reagent | Hospira Inc. | NDC 0409-4277-01 | Used as the local anesthesia during surgeries. |
| Isoflurane | Reagent | Baxter Healthcare Corp. | NDC 10019-360-40 | Used as a general inhalation anesthetic drug during surgeries and as a general anesthesia during experiments with mice. |
| Alpha Chloralose | Reagent | Sigma | C0128 | Used as a general anesthesia during experiments with rats. |
| Fluorescein isothio-cyanate–dextran | Reagent | Sigma | FD2000S | Administrated to create ~ 500 nM concentration in blood. |
References
- Kleinfeld, D., Friedman, B., Lyden, P. D., Shih, A. Y., Chen, J., Xu, Z., Xu, X., Zhang, J. Targeted occlusion to surface and deep vessels in neocortex via linear and nonlinear optical absorption, Animal Models of Acute Neurological Injuries. Contemporary Neuroscience Series. , (2007).
- Mostany, R., Portera-Cailliau, C. A Craniotomy Surgery Procedure for Chronic Brain Imaging. J Vis Exp. , (2008).
- Lebedev, A. Y., Cheprakov, A. V., Sakadzic, S., Boas, D. A., Wilson, D. F., Vinogradov, S. A., A, S. Dendritic Phosphorescent Probes for Oxygen Imaging in Biological Systems. Applied Materials & Interfaces. , (2009).
- Finikova, O. S., Lebedev, A. Y., Aprelev, A., Troxler, T., Gao, F., Garnacho, C., Muro, S., Hochstrasser, R. M., Vinogradov, S. A. Oxygen microscopy by two-photon-excited phosphorescence. Chemphyschem. 9, 1673-1679 (2008).
- Sakadžić, S., Yuan, S., Dilekoz, E., Ruvinskaya, S., Vinogradov, S. A., Ayata, C., Boas, D. A. Simultaneous imaging of cerebral partial pressure of oxygen and blood flow during functional activation and cortical spreading depression. Appl Opt. 48, D169-D177 (2009).
- Yaseen, M. A., Srinivasan, V. J., Sakadzic, S., Wu, W., Ruvinskaya, S., Vinogradov, S. A., Boas, D. A. Optical monitoring of oxygen tension in cortical microvessels with confocal microscopy. Opt Express. 17, 22341-22350 (2009).
- Sakadzic, S., Roussakis, E., Yaseen, M. A., Mandeville, E. T., Srinivasan, V. J., Arai, K., Ruvinskaya, S., Devor, A., Lo, E. H., Vinogradov, S. A., Boas, D. A. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat Methods. 7, 755-759 (2010).

