Bioelectric Analyses of an Osseointegrated Intelligent Implant Design System for Amputees
Summary
There is a need to develop alternative prosthesis attachment due to limb loss attributed to vascular occlusive diseases and trauma. The goal of the work is to introduce an osseointegrated intelligent implant design system to increase skeletal fixation and reduce periprosthetic infection rates for patients needing osseointegrated technology.
Abstract
Protocol
Part 1: Using Computed Tomography (CT) Scans for Amputee Reconstruction
- Retrospective CT scans were collected from the University of Utah and Department of Veteran Affairs hospitals after obtaining IRB and HIPAA approval.
- CT scans were selected because they allow clear distinction between tissue types based on x-ray absorbency.
- CTs were manually inspected and included in the study based on the absence of metal implants to prevent image artifact.
Part 2: Model Generation with Seg3D
- Files were downloaded as Dicom images and loaded into Seg3D (version 1.11.0, software.sci.utah.edu) as a new volume.
- A median filter was used to smooth the imported volumes prior to determining geometrically defined tissue structures.
- The tissue boundaries of the bone, bone marrow, organs and adipose tissue were generated by thresholding the CT files interactively (Figure 1).
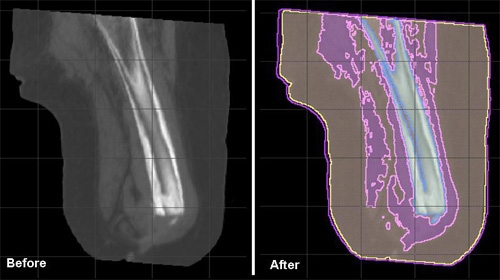
Figure 1: A sagittal cross section of an amputee residual limb thresholded and separated into specific tissue types. - The musculature was obtained by manually setting seed points inside the thresholded muscle tissue and using a confidence connected filter to find all the tissue connected to the seed points. This step eliminated erroneous tissues which may have been grouped together with the muscle based on similar absorbency from CTs.
- The skin, which was impossible to discern reliably from the CT images, was generated by dilating the outermost tissue 2 millimeters based on average skin thickness to produce a layer of homogeneous thickness that surrounded the full model1.
- Segmentations were manually inspected, corrected to ensure accuracy and combined in a hierarchy into a single label map required for finite element analysis (Figure 1).
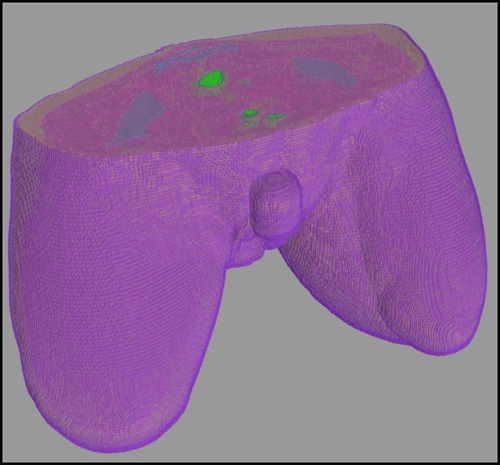
Figure 2: Representative hierarchical model of a bilateral amputee created with Seg3D.
Part 3: Preparation for Finite Element Analysis
- A 10 cm implant was designed in Matlab to serve as the implanted orthopedic device and cathode for electrical stimulation and imported into SCIRun (version 4.0, software.sci.utah.edu).
Part 4: Electrode Placement & Design
- SCIRun was utilized for electrode design because it supports interactive electrode placement and simulation.
- A network was created and modules organized with specific functions to generate the mesh (Figure 3). Modules were important for defining boundary conditions, tissue conductivities, mesh refinements, generating Matlab histograms, recording field data, etc (Table 1).
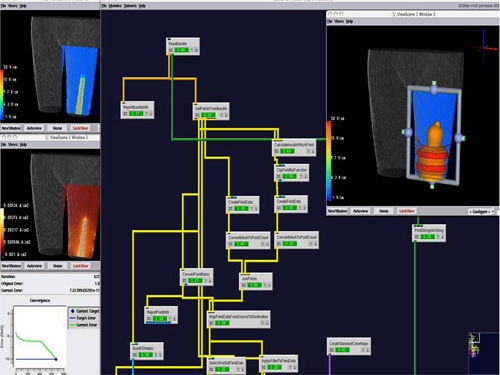
Figure 3: Representative network image from a pilot study using a two band external electrode configuration.
Table 1 Conductivities Assigned to Segmented Tissues Tissue Type Conductivities [S/m] Organ 0.22 Skin 0.26 Adipose 0.09 Muscle 0.25 Cortical Bone 0.02 Bone Marrow 0.07 - The configurations for the electrodes consisted of a one patch electrode, two patch electrodes, one continuous band and two continuous bands.
- External electrode bands were applied to the residual limb of the models generated from patient CT scans and were 1.6 cm in thickness.
- Electrode patches were placed as a strip covering approximately half the diameter of the residual limb and were 3 cm in thickness.
- The inner cortical implant which represented the osseointegrated implant was set to endosteal diameter to allow for perfect implant fit and fill2.
Part 5: Finite Element Analysis
- The simulations were generated assuming that the electric metrics could be calculated using a quasi-static approach with no time dependency.
- The model was computed by solving Laplace’s equation for each tissue type generated from the Seg3D segmentations.
- The boundary conditions were formed by the electrodes that injected currents and the guideline that current remained within the body.
- Since the electrodes and the implant had a much larger conductivity than the surrounding tissues, it was assumed that the implant (cathode) was at a constant potential, likewise the surface electrodes were modeled with a constant potential difference from the percutaneous implant.
- To evaluate the efficacy of electrode configuration and sizing, patient specific models were developed and the electrical potential around the implant interface was used to determine localized field strengths.
- The model was generated using a hexahedral mesh that consisted of approximately 1.8 million elements which were treated as piecewise homogenous, ohmic and isotropic.
- The optimal model for this experiment was selected with a relative difference <5% in voltage gradients confirmed with a mesh sensitivity study to insure model accuracy (Table 2).
Table 2 Mesh Sensitivity Study for Amputee Model Mesh Elements Nodes Relative Difference 100 100 50 149089 161131 0.0995 125 125 75 350180 371472 0.0802 150 150 100 673032 706082 0.0545 175 175 125 1146778 1194044 0.0527 200 200 150 1796690 1860772 0.0439 250 250 200 3745038 3850202 0.0364 275 275 225 5097243 5226587 0.0301 300 300 250 6742588 6898729 0.0000 - Using an iterative solver, the electric metrics in the finite element models were computed for the electrode configurations.
Discussion
Understanding the Electrical Stimulation Paradigm
Enhancements in medical care and evacuation strategies on the field of combat have lead to an increased number of warriors surviving disastrous war related injuries. While the improved survival rate is a medical advancement, servicemen and women are returning from combat with amputations that require intensive follow-up care, extensive rehabilitation and expensive prosthetic services from the Veteran Affairs Health Care System3. Congressional reports detail that over 1,000 war-related amputations have occurred as a result of Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) conflicts4.
In the case of OEF and OIF veterans, approximately 15% of returning warriors have lost multiple limbs and a significant number of returning servicemen and women have short residual limbs where socket technology is not an option or has been rejected by the patient. The reported discontinued use of upper extremity prosthesis even exceeds 50% because the fixation devices are cumbersome and difficult to use comfortably5. Lower extremity prostheses are equally problematic and the common problems associated with soft tissue sockets include the inability to walk on challenging terrain6, limited residual limb length7, patient discomfort5, concern with non-physiological loading8, irritation from heterotopic ossification9 and risk of debilitating diseases10. However, osseointegration technology is a novel surgical technique that may reduce pain11, skin irritation12, enhance osseoperception13, improve mobility6, decrease pressure sores associated with sockets6, reduce energy for ambulation7,14 and better serve veteran and warriors with limited residual limb length15.
Despite the numerous physical and psychological advantages of osseointegration, the associated surgical procedures require more advanced infection prevention treatment streategies16, require long rehabilitation programs and include restrictive weight bearing protocols which may last up to 1.5 years postoperative17. Because the viability of the host bone and length of the residual limb is important for muscle attachment and functionality, developing new devices to improve osseointegration is key for returning servicemen and women. Therefore, development of an osseointegrated intelligent implant design (OIID) system which uses controlled electrical stimulation may reduce the length of rehabilitation and increase skeletal attachment for veteran and warrior amputees. However, since no current device is commercially available and directed for use with percutaneous osseointegrated implants, the motivation of the program is to confirm safety and efficacy with finite element analysis.
Understanding the role of electrical stimulation in bone remodeling, specifically the deposition of osteoids and mineralization, has remained speculative. However, electrical activity observed in bone may be the result of mechanical loading18,19 and therefore an electrical stimulus may be an effective mechanism for inducing bone repair19. The logic behind the hypothesis is explained in a fracture healing model. When long bones are loaded, the side in tension becomes electropositive and the compression side electronegative20,21, however, once a bone is broken, the site will remain electronegative with respect to the surrounding environment until healing has commenced and homeostasis resumed21. Simulating the natural healing cascade with an electrical signal has been believed to assist with calcium deposition22, slight alterations in oxygen content and pH23, recruitment of growth factors22 and assists with osteoblast migration and secretion of additional extracellular matrix24.
The premise that electrical stimulation alone could govern complete bone repair has been redefined and the current new hypothesis proposes that complete unions are formed by mechanical loads and an electrical stimulation co-stimulus19. The electrical impulses observed in vivo are associated with piezoelectric deformation of collagen or the large electro-kinetic currents produced by ionic constituents flowing past mineral portions of the bone matrix25. In fact, spontaneous potentials have been reported in bone as large as 6 millivolts and correlates with an increase in the mineral apposition rate of bone26.
Early work by Brighton and Friedenberg18,21,27,28 used the concept of electrical stimulation for bone regeneration in the 1960s and 1970s and demonstrated that direct current could be used to repair non-unions in a shorter period of time when compared to traditional healing methods. Additional models have investigated bone formation with restrictive weight bearing and demonstrated a thirty-one percent increase in osteogenic activity between controls and electrically stimulated limbs25.
While researchers in the field of electrical stimulation have paved the way for understanding the mechanism for osteoblast matrix deposition with electrical stimulation, inadequate understanding has limited the expansion of this technology. While there are many cases of successful healing of non-unions and fracture healing models, examples of patient discomfort and failed attempts are replete in the literature as well29. The problem with electrical stimulation occurs from scientists and clinicians controlling the wrong electric metrics and concentrating solely on current magnitudes. Previous researchers have looked to current as the “magic bullet” to fixing the approximate 500,000 non-unions which occur annually30. However, repeatability between models has been limited from joule heating complications31 and not determining current densities32. In fact, all manufactured biomedical devices must be limited to a current density less than 2 mA/cm2 as outlined by the International Electrotechnical Commission to prevent localized tissue necrosis and patient discomfort33.
Aside from assisting with skeletal fixation, controlled electrical stimulation may also prevent bacterial adhesion on orthopedic implants and reduce the risk for osteomyelitis and biofilm formation34-37. Biofilm formation on orthopedic devices lead to patient complications and significant distress for those who depend on these devices38. Emphasis is placed on the necessity to have completely sterilized instrumentation and implants prior to surgery39 , however it is often difficult to diagnose bacteria adhesion as evident from many negative cultured cases which indeed are infected40. This problem is often coupled with the fact that biofilms are slow growing in nature40, cannot be growth accurately in vitro39, depend on the type of bacteria cells, cleanliness of the surface and the immune system of the affected person39. Investigation of European transfemoral amputees with oseeointegration technology reveal the most frequent problem is infection (frequent superficial infections, 1/3 periprosthetic infections)41. While there have been vast improvements in surgical preparation, eradicating bacteria is one of the fundamental factors for improving osseointegration since biofilms are between 500-5000 thousand times more difficult to eradicate due to their non-platonic form34,35,39. Therefore, utilizing electrical stimulation as a modality for removing harmful bacterial colonies and increasing skeletal fixation are important factors for ensuring the protection of patient health and OIID efficacy.
The advantages of using veteran and warrior amputees are that the relative youth and otherwise good health of these individuals make them an ideal population for aggressive rehabilitation and a percutaneous post will serve as an ambulatory aid and may be developed as an exposed cathode for electrical stimulation. The presence of an osseointegrated implant does not require additional surgical procedures to insert electrical components, allows the device to be controlled externally and prevents further risk of infection42. Therefore, by understanding the method of current injection into the residual limb of veteran and warrior amputees, an electric field on the magnitude of 1-10 V/cm may be established, controlled and measured at the implant interface. It is hypothesized that this will allow safe levels of electricity to be delivered, capable of inducing osteoblast migration and improving skeletal attachment. An electric field of this degree will increase the quantity and quality of bone at the implant interface, and improve the prospects for accelerated rehabilitation and skeletal fixation for an amputee. Use of electrical stimulation has not been investigated as a modality to accelerate osseointegration in an intramedullary prosthetic implant and presents numerous opportunities for translational research to improve patient care.
Experimental Results
The necessity for patient specific models with a percutaneous electrical stimulation device was supported in the study. The simulations developed for the proposed biomedical device may have the capabilities of expediting skeletal attachment by increasing osteoblast migration and preventing bacterial adhesion27,34,36,39. Computation modeling has effectively shown that 1-10 V/cm electric fields and current densities below 2 mA/cm2 may be generated using the implant as a functional cathode and is most homogenously distributed using a two band external electrode. The OIID system may be the first step to resolving the classic problem associated with electrical stimulation; the inability to define current pathways in the human body43. Therefore, establishing tools for enhancing skeletal attachment may assist with reducing the length of rehabilitation required for an osseointegrated procedure.
Utilizing electrical stimulation for older amputees is also a critical aspect which must be explored as well. Bone mass is maximum a decade after skeletal growth ceases but decreases significantly by the eighth and ninth decade44. As long bones change with age, the endosteal diameter tends to increase more rapidly than the periosteal diameter which may lead to implant loosening45. This problem coupled with the reduction of strain on bones by weaker muscles may contribute to debilitating diseases such as osteoporosis and osteopenia45 and require additional treatment options for patients with osseointegrated implants. However, controlled electrical stimulation and mechanical loading may act as a synergistic catalyst of bone ongrowth and maintain host bone bed integrity with elderly patients using an OIID system.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This material is based upon research supported (or supported in part by) the Technology Commercialization Office, Salt Lake City, UT, Office of Research and Development, Rehabilitation R&D Service, DVA SLC Health Care System, Salt Lake City, UT, Department of Defense PRMRP Grant (No. PR054520), the Albert & Margaret Hofmann Chair and the Department of Orthopaedics, University of Utah School of Medicine, Salt Lake City, UT; Technical support for the simulations was provided by the Center for Integrative Biomedical Computing of Scientific Computing and Imaging Institute and was made possible in part by software from the NIH/NCRR Center for Integrative Biomedical Computing, P41-RR12553-07.
Additional gratitude is extended to Gwenevere Shaw for assistance with manuscript preparation and Dustin Williams for the image of biofilm.
References
- Tortora, G. J., Nielsen, M. T., Roesch, B., et al. . Principles of Human Anatomy. , (2009).
- Bloebaum, R. D., Bachus, K. N., Momberger, N. G., Hofmann, A. A. . , (1993).
- Goldberg, M. S. . Military Medical/NBC Technology. 11 (8), 31 (2007).
- Fischer, H. . Report No. Order Code RS22452. , (2008).
- Moore, T. J., et al. . Clin Orthop Relat Res. 238, 219 (1989).
- Hagberg, K., Branemark, R. . Prosthet Orthot Int. 25 (3), 186 (2001).
- Todd, T. W., Barber, C. G. . J Bone Joint Surg Am. 16, 53 (1934).
- Jaegers, S. M., Arendzen, J. H., de Jongh, H. J. . Archives of Physical Medicine and Rehabilitation. 76 (8), 736 (1995).
- Potter, B. K., et al. . Journal of American Academy of Orthopaedic Surgeons. 14 (10), 191 (2006).
- Kulkarni, J., Adams, J., Thomas, E., Silman, A. . Clin Rehabil. 12 (4), 348 (1998).
- Smith, D. G., et al. . Clin Orthop Relat Res. (361), 29 (1999).
- Pasquina, P. F., et al. . Archives of Physical Medicine and Rehabilitation. 87 (3), 34 (2006).
- Ysander, M., Branemark, R., Olmarker, K., Myers, R. R. . Journal of Rehabilitation Research & Development. 38 (2), 183 (2001).
- Couch, N. P., David, J. K., Tilney, N. L., Crane, C., et al. . American Journal of Surgery. 133 (4), 469 (1977).
- Morgenroth, D. C., Shakir, A., Orendurff, M. S., Czerniecki, J. M. . Am J Phys Med Rehabil. 88 (2), 108 (2009).
- Pendegrass, C. J., et al. . Journal of Bone and Joint Surgery British. 90 (1), 114 (2008).
- Lee, W. C., et al. . Med Eng Phys. 30 (7), 825 (2008).
- Brighton, C. T., Friedenberg, Z. B., Zemsky, L. M., Pollis, P. R. . J Bone Joint Surg Am. 57 (3), 368 (1975).
- Spadaro, J. A. . Bioelectromagnetics. 18 (3), 193 (1997).
- Brighton, C. T., Friedenberg, Z. B., Mitchell, E. I., Booth, R. E. . Clin Orthop Relat Res. 124, 2 (1976).
- Friedenberg, Z. B., Brighton, C. T. . J Bone Joint Surg Am. 48 (5), 915 (1966).
- Yonemori, K., et al. . Bone. 19 (2), 173 (1996).
- Treharne, R. W., Brighton, C. T., Korostoff, E., Pollack, S. R. . Clin Orthop Relat Res. (145), 300 (1979).
- Wiesmann, H., et al. . Biochimica et Biophysica Acta. 1538 (1), 28 (2001).
- McLeod, K. J., Rubin, C. T. . J Bone Joint Surg Am. 74 (6), 920 (1992).
- Rubinacci, A., Tessari, L. . Calcified Tissue International. 35 (6), 728 (1983).
- Brighton, C. T., et al. . J. Bone Joint Surg Am. 63 (5), 847 (1981).
- Friedenberg, Z. B., Zemsky, L. M., Pollis, R. P., Brighton, C. T. . J Bone Joint Surg Am. 56 (5), 1023 (1974).
- Jorgensen, T. E. . Clin Orthop Relat Res. 124, 124 (1977).
- Ehrlich, G. D., et al. . Clin Orthop Relat Res. 437, 59 (2005).
- Soong, H. K., et al. . Investigative Ophthalmology & Visual Science. 31 (11), 2278 (1990).
- Li, W. P., et al. . Bone. 32 (8), 986 (2006).
- Leitgeb, N., Cech, R., Schrottner, J. . Radiat Prot Dosimetry. 124 (2), 124 (2007).
- van der Borden, A. J., et al. . Biomaterials. 28 (12), 2122 (2007).
- van der Borden, A. J., van der Mei, H. C., Busscher, H. J. . Biomaterials. 26 (33), (2005).
- Costerton, J. W., et al. . Annual Review of Microbiology. 41, 435 (1987).
- Neut, D., van der Mei, H. C., Bulstra, S. K., Busscher, H. J. . Acta Orthop. 78 (3), 299 (2007).
- Anwar, H., Dasgupta, M. K., Costerton, J. W. . Antimicrobial Agents and Chemotherapy. 34 (11), 2043 (1990).
- Costerton, J. W. . Clin Orthop Relat Res. (437), 7 (2005).
- Nelson, C. L., et al. . Clin Orthop Relat Res. 437, 25 (2005).
- Gunterberg, B., et al. . , (1998).
- Lavine, L. S., Grodzinsky, A. J. . J Bone Joint Surg Am. 69 (4), 626 (1987).
- Chakkalakal, D. A., Johnson, M. W. . Clin Orthop Relat Res). (161), 133 (1981).
- Buckwalter, J. A., Glimcher, M. J., Cooper, R. R., Recker, R. . J Bone Joint Surg Am. 77 (2), 1276 (1995).
- Lane, J. M., Vigorita, V. J. . J Bone Joint Surg Am. 65 (2), 274 (1983).

