Enhanced Cochlear Coverage and Hearing Preservation in High-Frequency Hearing Loss via Electric Acoustic Stimulation with Longer Electrode
Summary
Electric acoustic stimulation (EAS) with longer electrodes can offer broader cochlear coverage and various types of maps in cases of high-frequency hearing loss. Combining less invasive surgery, flexible lateral-wall electrodes, and steroid administration permits deeper insertion with little or no surgical trauma, resulting in good preservation of hearing.
Abstract
Electric-acoustic stimulation (EAS) is a promising treatment to improve hearing ability in patients with high-frequency hearing loss (HL). In EAS surgeries, shorter electrodes have been preferred to avoid the presence of an electrode covering the residual hearing region. However, our earlier studies showed that EAS with longer electrodes (28 mm) could preserve acoustic hearing. Additionally, we reported that the hearing preservation (HP) scores were independent of the length of the inserted electrodes, consistent with the systematic review. As most EAS patients gradually lose residual hearing over time due to the natural course of HL, in these cases, providing broader cochlear coverage using longer electrodes was beneficial toward better place-pitch matching. In addition to preparing for the deterioration in hearing in the future, EAS with longer electrodes could offer various types of map strategies. Herein, we show the pre-, intra-, and post-procedures for EAS surgery. Appropriate preoperative evaluation, less invasive surgery, flexible lateral-wall electrodes, and steroid administration resulted in good HP following EAS with longer electrodes.
Introduction
Conventional cochlear implantation (CI) is a standard treatment for improving hearing ability in patients with severe-to-profound hearing loss (HL). Subsequently, electric-acoustic stimulation (EAS) is used to treat patients with severe high-frequency hearing loss and residual low-frequency hearing1. In such patients, preserving the residual acoustic hearing is important to achieve better speech perception in noise, sound localization, and improved sound quality when listening to music2. To address this, the use of shorter electrodes in EAS patients has been preferred to avoid interfering with the residual function in the acoustic region of the cochlea. However, our earlier studies3,4,5,6 documented that even in EAS cases, less invasive CI surgery combined with thin, straight, and flexible "longer" electrodes enabled the preservation of residual hearing. Additionally, we reported that hearing preservation was unrelated to the length of the inserted cochlear implant electrode7, consistent with the systematic review8.
In patients who underwent conventional CI, longer electrodes led to better speech perception9,10,11 as longer electrodes provide broader cochlear coverage and better place-pitch matching. Similarly, a correlation between a deeper angle of insertion (AID) and better hearing was reported12,13. In most EAS patients, the residual hearing gradually deteriorates over time14. Identifying the responsible gene for HL allows one to predict future hearing. When it is anticipated that their hearing would be lost across all frequencies in the future, EAS with longer electrodes, not shorter electrodes, is ideal in providing a higher percentage of cochlear coverage15. The optimal AID was thought to range from 630° to 720°16,17,18, corresponding to the distribution of spiral ganglion neurons in the human cochlea. However, as each cochlear duct length (CDL) has a wide range of variation19, measuring the CDL in each case was required to achieve the appropriate AID, even in EAS patients. Recently, commercially available software (see Table of Materials) allowed the measurment of each CDL easily based on computed tomography (CT) data, which was clinically feasible.
This protocol describes the following: (1) a preoperative evaluation that included genetic analysis to identify the etiology of HL and cochlear duct length measurement to determine the optimal electrode length, (2) a less invasive surgical procedure via exoscopy and endoscopy, and (3) the postoperative hearing outcome and mapping strategy in patients with high-frequency HL who underwent EAS with a longer electrode.
Protocol
The described procedures were approved by the Institutional Review Board of Shinshu University School of Medicine (Approval no. 4133). The patients provided written informed consent before participating in the study. The reagents, equipment, and software used for this study are listed in the Table of Materials.
1. Preoperative evaluation
- Review the series of pure-tone audiograms in patients with residual hearing to ascertain the progression of HL in each case.
- Conduct genetic testing on blood samples obtained from both patients and their family members.
- Recommend to perform next-generation sequencing on a panel of genes associated with HL6,7.
- Conduct imaging tests, such as CT and/or magnetic resonance imaging (MRI). It was recommended that the CT slice thickness be 0.6 mm or less. For MRI, a strength of 1.5 Tesla was deemed sufficient.
- Make predictions regarding future residual acoustic hearing to the best extent possible following the collection of the audiogram series6 and the identification of etiology through imaging and/or genetic analysis.
- Import DICOM data from preoperative CT images into the OTOPLAN software. The software automatically measures the CDL in each case.
- Select the appropriate length of the CI array to cover the region where residual hearing is anticipated to deteriorate in the future. Employ only thin, straight, and flexible electrodes.
- Administer prednisolone orally at a dosage of 0.5-1.0 mg/kg/day beginning 2 days prior to surgery.
2. Surgical procedure
- Place the patient in the supine position.
- Induce anesthesia using intravenous fentanyl (1-2 µg/kg), propofol (1-2 mg/kg), and rocuronium (0.6 mg/kg). Maintain anesthesia with propofol (3-8 mg/kg/h), remifentanil (0.1-0.2 µg/kg/min), and fentanyl (total: 300-500 µg), without vecuronium, to facilitate neuromuscular monitoring.
- Administer an intravenous infusion of 8 mg of dexamethasone 30 min before making the incision.
- Rotate the patient's head to 45 degrees during surgery.
- Inject local anesthesia (subcutaneous 0.5% lidocaine with 1:1,00,000 epinephrine) postauricularly.
- Perform a postauricular incision of 5-6 cm (lazy S-shaped) and mastoidectomy with 6.0-4.0 mm cutting bars until reaching the point where the antrum includes the lateral semicircular canal and the short process of the incus6.
- Perform a posterior tympanotomy between the expected facial nerve and chorda tympani using 1.5-2.0 mm diamond bars.
- Visualize the round window niche and remove the bony overhang of the round window using a low-speed drill with a 1.0-1.5 mm diamond bar, exposing the round window membrane.
- Sufficiently open the round window membrane using a pick and carefully insert the electrode slowly for over 3 min.
- Perform X-ray and audiograms after completing the electrode insertion.
3. Postoperative evaluation
- Administer intravenous dexamethasone at 8 mg/day, 4 mg/day, and 4 mg/day for 3 days following surgery.
- Measure unaided auditory thresholds 6 months after the initial activation, and assess the HP rate using the classification provided by Skarzynski et al.20.
- Optimize map settings using a software tool based on the results of the postoperative audiogram and patient preferences.
Representative Results
EAS was conducted on 10 patients (11 ears) who fulfilled the audiological criteria for EAS (refer to Table 1). The inclusion criteria comprised the following: bilateral pure-tone hearing levels ≤65 dBHL for 125 Hz, 250 Hz, and 500 Hz; ≥80 dBHL at 2000 Hz; and ≥85 dBHL at 4000 Hz and 8000 Hz. Additionally, minimal benefit from conventional hearing aids was required, defined as monosyllable scores in quiet below 60% even in the optimal aided condition. All participants underwent EAS using longer electrodes (see Table of Materials). Figure 1 shows the preoperative and 6-month postoperative audiograms following EAS surgery, suggesting that residual low-frequency hearing was well-preserved in all cases. According to the hearing preservation (HP) classification system reported by Skarzynski et al.20, 36.4% (4 of 11 cases) showed complete HP, and 63.6% (7 of 11 cases) showed partial HP. There were no cases of significant hearing deterioration.
Case presentation (Case #8)
At the age of 5, during a routine elementary school wellness check-up, a 14-year-old girl who had not undergone newborn hearing screening was flagged for suspected hearing loss (HL). Subsequently diagnosed with high-frequency HL, she began using hearing aids. Given the observed deterioration in her hearing, she sought evaluation at our department at the age of 13.
Pathogenic variants of the CDH23 gene were identified via genetic testing6. Since the residual hearing was likely to deteriorate due to CDH23-related HL, a longer electrode was selected to cover the acoustic region. She underwent EAS surgery on the left ear at the age of 14. The residual hearing was completely preserved 6 months after surgery. She preferred "electric-stimulation (ES)-only mapping" with activated apical electrodes traversing the region of residual hearing at that time (Figure 2). The patient was satisfied with the acoustic amplification obtained using ES at low frequencies.
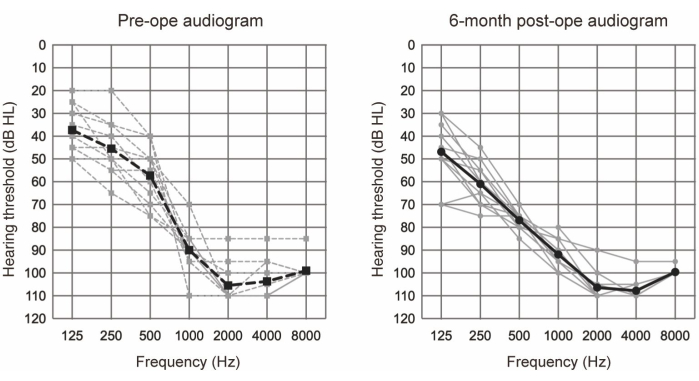
Figure 1: Average air-conduction hearing thresholds. The dashed and solid lines indicate preoperative and 6-month postoperative measurements, respectively. Gray and black lines show the individual data and the mean, respectively. This figure has been adapted from Yoshimura et al.6. Please click here to view a larger version of this figure.
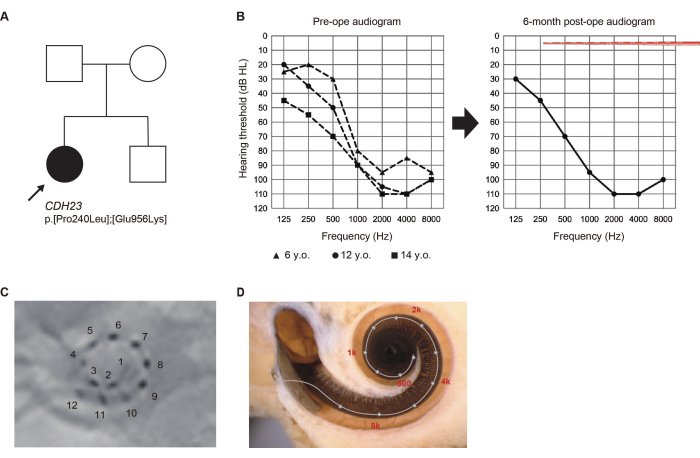
Figure 2: Clinical findings of Case #8. (A) Pedigree of the patient. (B) Preoperative and 6-month postoperative audiograms. Electrode array illustrations represent insertion depth. (C) Postoperative X-ray findings, with numbers corresponding to individual channels. (D) Imaging for each electrode location and the reference tonotopic map. This figure has been adapted from Yoshimura et al.6. Please click here to view a larger version of this figure.
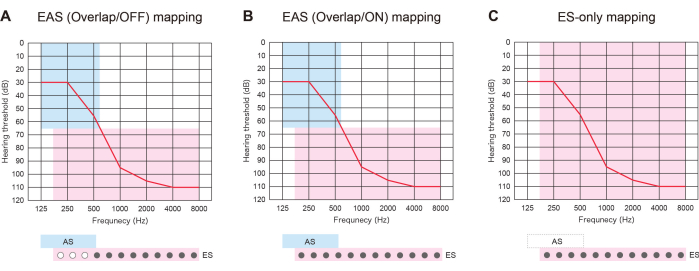
Figure 3: Three types of mapping strategy in patients with EAS using longer electrodes. (A) EAS (Overlap/OFF) map. Some of the apical contacts were deactivated, while the acoustic stimulation (AS) was activated. (B) EAS (Overlap/ON) map. Both electrical stimulation (ES) in the cochlear implantation (CI) default setting and AS were utilized. (C) ES-only map. In the ES setting, the frequency range was set from 70-8,500 Hz. AS was deactivated. Please click here to view a larger version of this figure.
| Patient | Implanted | Implanted | Responsible | Pre-op | 6M post-op | HP numerical | HP | |
| No. | Gender | age (years) | side | Gene | LFA (dB) | LFA (dB) | scale (%) | classification |
| 1 | F | 12 | L | SLC26A4 | 63.3 | 70 | 70.8 | Partial |
| 2 | F | 9 | L | SLC26A4 | 50 | 65 | 62.5 | Partial |
| 3 | M | 50 | L | CDH23 | 50 | 56.7 | 78.6 | Complete |
| 4 | F | 31 | L | Unknown | 45 | 61.7 | 67.7 | Partial |
| 31 | R | 46.7 | 56.7 | 75.9 | Complete | |||
| 5 | F | 57 | R | Unknown | 38.3 | 65 | 59 | Partial |
| 6 | F | 55 | R | Unknown | 26.7 | 55 | 68.4 | Partial |
| 7 | M | 21 | R | LOXHD1 | 33.3 | 66.7 | 37.2 | Partial |
| 8 | F | 14 | R | CDH23 | 56.7 | 48.3 | 100 | Complete |
| 9 | M | 20 | L | Unknown | 51.7 | 73.3 | 48.1 | Partial |
| 10 | M | 64 | R | Unknown | 51.7 | 58.3 | 82.1 | Complete |
Table 1: Summary of subject characteristics and hearing preservation outcomes. This table has been adapted from Yoshimura et al.6.
Discussion
Identifying the etiology of HL in the preoperative evaluation is crucial for predicting the future audiogram in each case. In our earlier study, pathogenic variants in the CDH23, ACTG1, Mit1555A>G, MYO7A, MYO15A, SLC26A4, and TMPRSS3 genes were frequently identified in patients with high-frequency HL7. In most of these patients, the residual hearing gradually deteriorated (see CDH23-related HL case in Figure 2). In these cases, the natural course of HL was considered when selecting the CI electrode for broader cochlear coverage.
Determining the length of the CI array required measuring the cochlear duct length (CDL). To facilitate this process, the OTOPLAN software proved to be clinically feasible21. In earlier versions, such as OTOPLAN 3.0, the CDL was manually measured to plot each cochlea's diameter, width, and height. However, with OTOPLAN 4.0, automatic measurement of the CDL became possible, offering increased convenience for surgeons and minimizing measurement variation. Notably, CT images with a slice thickness of 0.6 mm or less were suitable for the automatic analysis provided by OTOPLAN. Subsequently, the length of the CI array should be selected to achieve the optimal angle of insertion depth (AID) in each case.
To perform a less invasive EAS surgery, pre-, intra-, and post-steroid administration was required to minimize the acute inflammation that could elevate the hearing threshold22. In the near future, to minimize not only acute but also chronic reactions following CI and EAS surgery, the use of dexamethasone-eluting electrodes will be desirable. In addition to steroid administration, flexible lateral-wall electrodes were essential for minimizing trauma to the cochlea. Inserting such electrodes into the cochlea carefully and slowly using the round window approach resulted in minimal invasiveness. To aid in this, performing surgery using exoscopes and endoscopes was useful for generating a clearer field of view and confirming the tiny components of the middle ear. To prevent inducing extensive fibro-osseous tissue formation in the cochlea, the extended round window approach and cochleostomy must be avoided23.
EAS with longer electrodes was useful not only to prepare for the future deterioration of HL but also to offer three types of map strategies. If the inserted electrodes overlap with the residual hearing region, patients with EAS can use ES with or without AS: "EAS (Overlap/ON) map" or "ES-only map." Alternatively, they can turn off some of the apical contacts and activate the AS: "EAS (Overlap/OFF) map." If hearing deteriorates, all the contacts can be later switched on to provide better pitch matching (Figure 3). All these findings show that EAS with longer electrodes allows users to optimize maps for more natural hearing.
Herein, it is shown that preoperative preparation and advanced surgical techniques are essential to minimize surgical trauma. Performing the above-named series of pre-and intra-operative procedures allowed patients to benefit from EAS with longer electrodes.
Limitations
Despite advances in the aforementioned less-invasive surgical procedures, residual hearing still deteriorates in a certain number of patients after CI. Intra-operative monitoring, such as cochlear microphonics (CM), would be clinically feasible to measure cochlear damage during the electrode insertion24. However, how to evaluate EAS with longer electrodes and what to do in the case of certain CM responses, such as a decrease in amplitude, remains unclear to date. Further studies in this area are required.
Declarações
The authors have nothing to disclose.
Acknowledgements
This research was funded by a Health and Labor Sciences Research Grant for Research on Rare and Intractable Diseases and Comprehensive Research on Disability Health and Welfare from the Ministry of Health, Labour and Welfare of Japan (S.U. 20FC1048, 23FC10149) and Grants-in-Aid from the Japan Agency for Medical Research and Development (AMED) (S.U. 19ek0109363h0002, 21ek0109542h003).
Materials
| DEXART 3.3 mg | Fuji Pharma | 22100AMX01404 | equal to dexamethasone sodium phosphate (4 mg) |
| DEXART 6.6 mg | Fuji Pharma | 22100AMX01402 | equal to dexamethasone sodium phosphate (8 mg) |
| Fentanyl injection 0.1 mg | TERUMO | 22100AMX00009 | |
| MAESTRO 7.0 | MED-EL | 4582290238456 | fitting software for map settings |
| Midas Rex MR8 | Medtronic | 301ADBZX00046000 | high speed drill |
| OTOPLAN software | Cascination / MED-EL | REF 20125 | for measuring cochlear duct length (CDL) |
| Predonine tablets | Shionogi | 16000AMZ01740000 | |
| Propofol 1% 50 mL | Maruishi Pharmaceutical Co.,Ltd | 30100AMX00158 | |
| Remifentanil 2 mg | Daiichi-Sankyo | 22800AMX00090 | |
| Rocuronium bromide 50 mg/50 mL | Maruishi Pharmaceutical Co.,Ltd | 22800AMX00534 | |
| SONNET2 EAS | MED-EL | 4582290241807 | processor |
| Synchrony2 FLEX28 | MED-EL | 4571573943026 | cochlear implant (electrode) |
| Xylocaine 0.5% with epinephrine | Sandoz Pharma | 4KUZ13127 |
Referências
- Von Ilberg, C., et al. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 61 (6), 334-340 (1999).
- Gstoettner, W., et al. A new electrode for residual hearing preservation in cochlear implantation: First clinical results. Acta Otolaryngol. 129 (4), 372-379 (2009).
- Moteki, H., et al. Feasibility of hearing preservation for residual hearing with longer cochlear implant electrodes. Acta Otolaryngol. 138 (12), 1080-1085 (2018).
- Usami, S., et al. Achievement of hearing preservation in the presence of an electrode covering the residual hearing region. Acta Otolaryngol. 131 (4), 405-412 (2011).
- Usami, S., et al. Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta Otolaryngol. 134 (7), 717-727 (2014).
- Yoshimura, H., Moteki, H., Nishio, S. Y., Usami, S. I. Electric-acoustic stimulation with longer electrodes for potential deterioration in low-frequency hearing. Acta Otolaryngol. 140 (8), 632-638 (2020).
- Yoshimura, H., et al. Genetic testing has the potential to impact hearing preservation following cochlear implantation. Acta Otolaryngol. 140 (6), 438-444 (2020).
- Van de Heyning, P. H., et al. Systematic literature review of hearing preservation rates in cochlear implantation associated with medium- and longer-length flexible lateral wall electrode arrays. Front Surg. 9, 893839 (2022).
- Buchman, C. A., et al. Influence of cochlear implant insertion depth on performance: A prospective randomized trial. Otol Neurotol. 35 (10), 1773-1779 (2014).
- Buchner, A., Illg, A., Majdani, O., Lenarz, T. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One. 12 (5), e0174900 (2017).
- Canfarotta, M. W., et al. Long-term influence of electrode array length on speech recognition in cochlear implant users. Laryngoscope. 131 (4), 892-897 (2021).
- Nassiri, A. M., et al. Hearing preservation outcomes using a precurved electrode array inserted with an external sheath. Otol Neurotol. 41 (1), 33-38 (2020).
- O’Connell, B. P., et al. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol. 37 (8), 1016-1023 (2016).
- Moteki, H., et al. Long-term results of hearing preservation cochlear implant surgery in patients with residual low frequency hearing. Acta Otolaryngol. 137 (5), 516-521 (2017).
- Von Ilberg, C. A., Baumann, U., Kiefer, J., Tillein, J., Adunka, O. F. Electric-acoustic stimulation of the auditory system: A review of the first decade. Audiol Neurootol. 16, 1-30 (2011).
- Ariyasu, L., Galey, F. R., Hilsinger, R., Byl, F. M. Computer-generated three-dimensional reconstruction of the cochlea. Otolaryngol Head Neck Surg. 100 (2), 87-91 (1989).
- Danielian, A., Ishiyama, G., Lopez, I. A., Ishiyama, A. Morphometric linear and angular measurements of the human cochlea in implant patients using 3-dimensional reconstruction. Hear Res. 386, 107874 (2020).
- Kawano, A., Seldon, H. L., Clark, G. M. Computer-aided three-dimensional reconstruction in human cochlear maps: Measurement of the lengths of organ of Corti, outer wall, inner wall, and Rosenthal’s canal. Ann Otol Rhinol Laryngol. 105 (9), 701-709 (1996).
- Rask-Andersen, H., et al. Human cochlea: Anatomical characteristics and their relevance for cochlear implantation. Anat Rec (Hoboken). 295 (11), 1791-1811 (2012).
- Skarzynski, H., et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol Suppl. 564, 3-13 (2013).
- Yoshimura, H., Watanabe, K., Nishio, S. Y., Takumi, Y., Usami, S. I. Determining optimal cochlear implant electrode array with OTOPLAN. Acta Otolaryngol. 143 (9), 748-752 (2023).
- Skarzynska, M. B., et al. Preservation of hearing following cochlear implantation using different steroid therapy regimens: a prospective clinical study. Med Sci Monit. 24, 2437-2445 (2018).
- Geerardyn, A., et al. Human histology after structure preservation cochlear implantation via round window insertion. Laryngoscope. 134 (2), 945-953 (2023).
- Campbell, L., et al. Intraoperative real-time cochlear response telemetry predicts hearing preservation in cochlear implantation. Otol Neurotol. 37 (4), 332-338 (2016).

.
